Final ID: LB23
Transauricular vagus nerve stimulation decreases inflammatory biomarkers and affects NIHSS in acute ischemic stroke patients: Results from the NUVISTA trial
Abstract Body: Objective: Neuroinflammation is an important element of acute ischemic stroke (AIS) pathology. We investigated if noninvasive transcutaneous auricular vagus nerve stimulation (taVNS) mitigates inflammation following AIS and explored its effect in clinical outcomes.
Methods: We conducted a randomized open-label pilot study, with blinded outcomes. Patients were randomized to taVNS stimulation every 12 hours vs sham stimulation. Inclusion criteria included: age≥18 years, acute anterior circulation large vessel occlusions (LVO), NIHSS≥6, pre-morbid modified Rankin score ≤2, <36 hours from symptom discovery, life expectancy >3 months, and no active cancer or immunosuppressive/modulating therapy, concomitant infections, hypotension, or bradycardia on arrival. Patients continued taVNS or sham stimulation for 5 days or until discharge, and received standard medical care. Primary endpoints included rate of change in inflammatory markers (white blood cell count and IL-1β, IL-6, TNF-α, IL-17, IL-10) from plasma taken on day 0, 1, 3, 5, and 7 of hospitalization. Secondary endpoints included rate of change in daily NIHSS and presence of safety endpoints (hypotension and bradycardia). We used multivariable mixed effects regression to examine the association between assigned treatment and continuous outcomes.
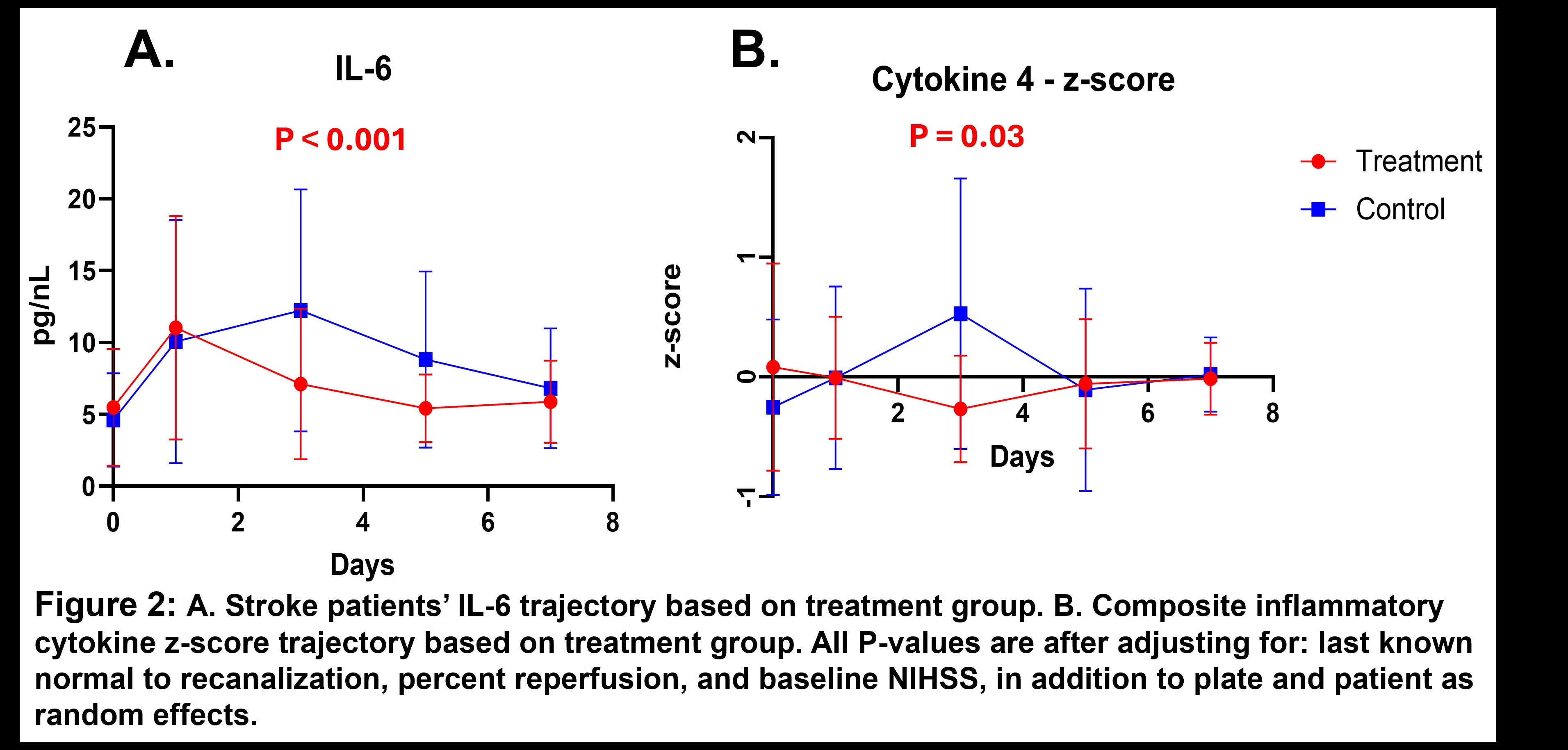
Results: We enrolled 35 patients (17 randomized to taVNS and 18 to sham). IL-6 and composite inflammatory cytokine z-score trajectories differed significantly between groups, with faster decline in the treated group (p<0.001 and p=0.03, respectively). We observed no significant difference between groups with regards to NIHSS as a whole (p=0.83), but NIHSS rate of change differed by laterality (interaction p=0.03), with a suggestion of faster decrease in patients treated with taVNS ipsilateral to the stroke (left) (0.527, 95% CI -0.35, 1.40 greater decrease per day in the treatment vs sham group, adjusted p=0.21). Development of hypotension, bradycardia, or infection did not differ significantly between treatment and sham groups (0%, 11.8%, and 17.7% in the treated group and 11.1%, 22.2%, and 16.7% in the control group, respectively, all p≥0.49)
Conclusions: taVNS alters inflammatory biomarkers in AIS patients with LVO, and is safe. There may be differential effects on clinical outcome based on the laterality of taVNS stimulation, but larger studies are needed to confirm these clinical findings.
Methods: We conducted a randomized open-label pilot study, with blinded outcomes. Patients were randomized to taVNS stimulation every 12 hours vs sham stimulation. Inclusion criteria included: age≥18 years, acute anterior circulation large vessel occlusions (LVO), NIHSS≥6, pre-morbid modified Rankin score ≤2, <36 hours from symptom discovery, life expectancy >3 months, and no active cancer or immunosuppressive/modulating therapy, concomitant infections, hypotension, or bradycardia on arrival. Patients continued taVNS or sham stimulation for 5 days or until discharge, and received standard medical care. Primary endpoints included rate of change in inflammatory markers (white blood cell count and IL-1β, IL-6, TNF-α, IL-17, IL-10) from plasma taken on day 0, 1, 3, 5, and 7 of hospitalization. Secondary endpoints included rate of change in daily NIHSS and presence of safety endpoints (hypotension and bradycardia). We used multivariable mixed effects regression to examine the association between assigned treatment and continuous outcomes.
Results: We enrolled 35 patients (17 randomized to taVNS and 18 to sham). IL-6 and composite inflammatory cytokine z-score trajectories differed significantly between groups, with faster decline in the treated group (p<0.001 and p=0.03, respectively). We observed no significant difference between groups with regards to NIHSS as a whole (p=0.83), but NIHSS rate of change differed by laterality (interaction p=0.03), with a suggestion of faster decrease in patients treated with taVNS ipsilateral to the stroke (left) (0.527, 95% CI -0.35, 1.40 greater decrease per day in the treatment vs sham group, adjusted p=0.21). Development of hypotension, bradycardia, or infection did not differ significantly between treatment and sham groups (0%, 11.8%, and 17.7% in the treated group and 11.1%, 22.2%, and 16.7% in the control group, respectively, all p≥0.49)
Conclusions: taVNS alters inflammatory biomarkers in AIS patients with LVO, and is safe. There may be differential effects on clinical outcome based on the laterality of taVNS stimulation, but larger studies are needed to confirm these clinical findings.
More abstracts on this topic:
84 Immune checkpoint profiling in major aortic diseases leads to identification of potential roles of CD155-CD206 pathway in suppressing inflammation and immune responses
Shao Ying, Saaoud Fatma, Xu Keman, Lu Yifan, Jiang Xiaohua, Wang Hong, Yang Xiaofeng
A Case of Transient Cortical Blindness occurring during Percutaneous Transluminal Coronary Angiography for Acute Coronary Syndrome.Adelakun Adeniyi, Farouji Iyad, Haddad Ahmad, Szwed Stanley
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)