Final ID: LB1
Procedural, safety, and functional outcomes following minimally invasive surgery for deep and lobar intracranial hemorrhages: MIND study results.
ICH is associated with the poorest long-term outcomes of any stroke subtype. ENRICH recently demonstrated that minimally invasive surgery (MIS) improves functional outcomes in patients with superficial ICH at 180 days.
Objective
The MIND study was designed to evaluate the safety and effectiveness of (MIS) with Artemis Neuro Evacuation Device and medical management (MM) compared to MM alone in patients with primarily deep and primarily lobar ICHs through 180 days.
Methods
MIND (NCT03342664) was a multicenter, open-label, randomized trial that enrolled patients with moderate-large (20-80 cc) supratentorial ICH treated within 24 hours of symptom onset. Participants were randomized (2:1) to MIS or MM alone with stratification for severity and ICH location. The primary effectiveness endpoint was a 180-day ordinal modified Rankin score (mRS). The primary safety endpoint was 30-day mortality.
Results
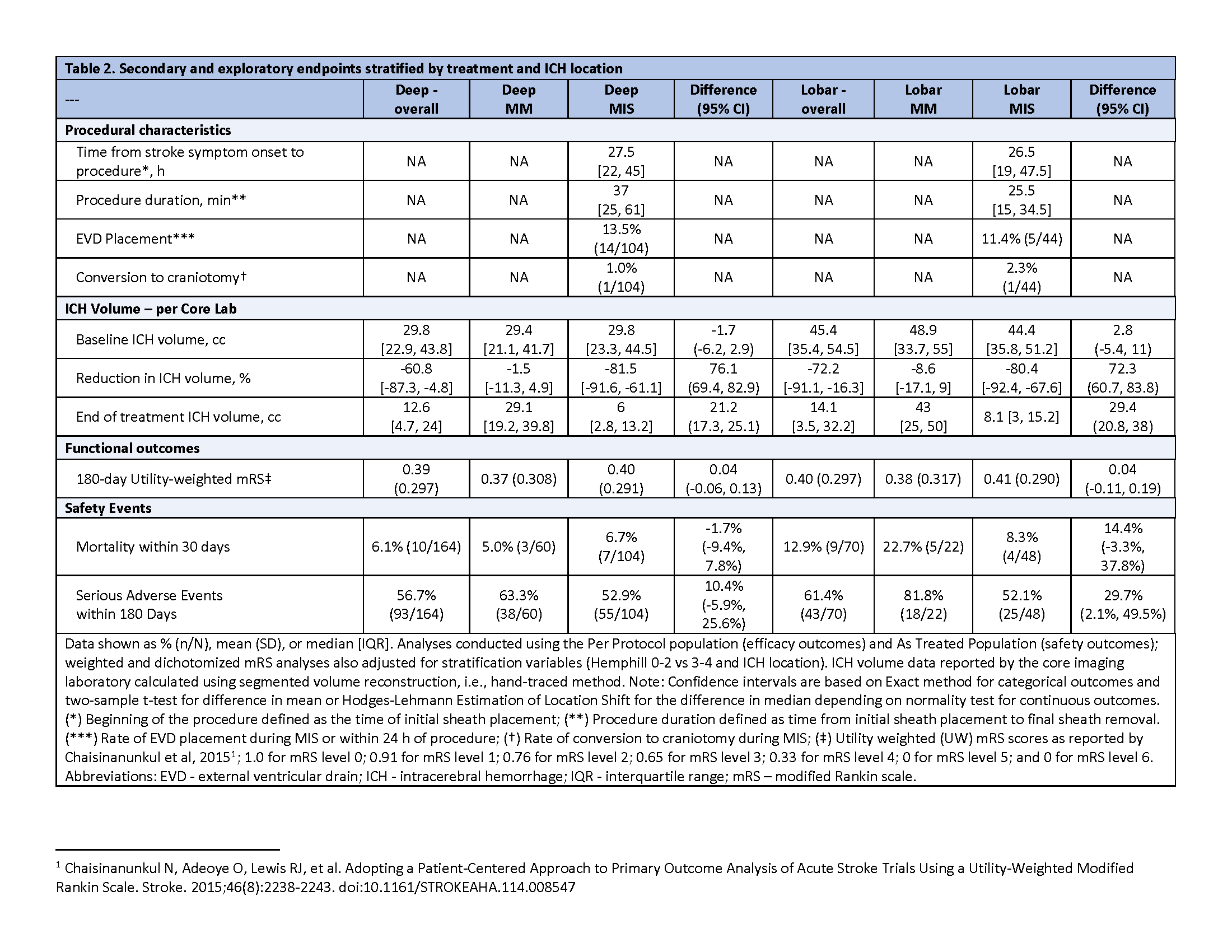
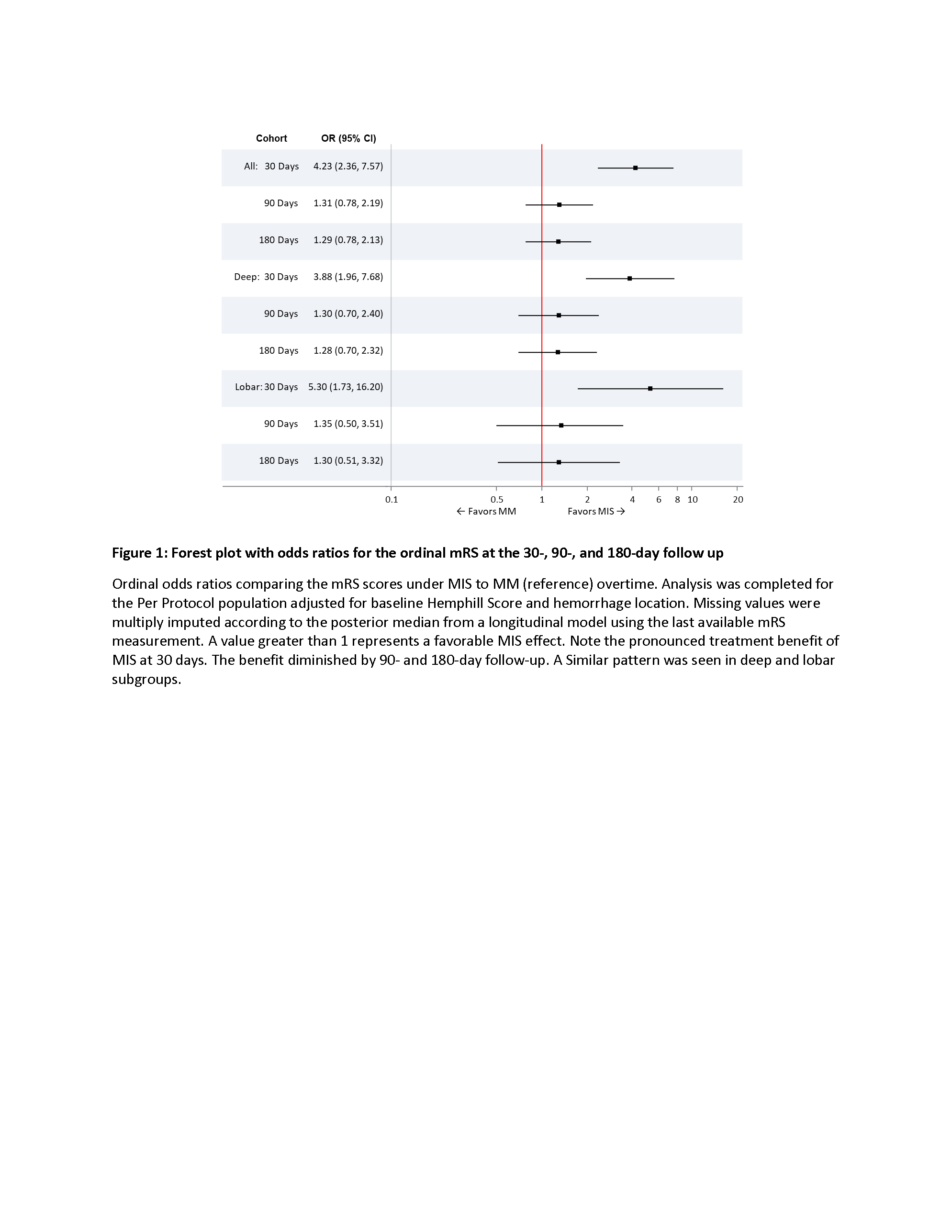
Given the positive ENRICH results and following an independent analysis, which concluded that randomization of only patients presenting with primarily deep hemorrhage was not feasible, study enrollment was stopped after 236 patients were enrolled. 167 (70.8%) presented with deep and 72 (29.2%) lobar ICH. Patients with deep ICH were younger (age 57 vs 69), less likely to be female (31.7 vs 48.6%), presented with more severe symptoms (NIHSS 19 vs 16; GCS 11 vs 13), and had more medical co-morbidities than patients with lobar ICHs. Deep ICHs tended to be smaller (35cc vs 50cc). Following MIS, ICH volume was reduced by 81.5% to 6.0 cc in the deep and 80.4% to 8.1cc in the lobar cohorts, respectively. MIS increased the odds of favorable clinical outcome (ordinal mRS) at 30 days in both cohorts; however, this benefit was no longer evident at 90 and 180 days. Overall, 30-day mortality was comparable following MIS (6.7% in deep vs 8.3% in lobar), however the rates in MM patients varied substantially by location (5.0% vs 22.7%, respectively). Deep and lobar cohorts had similar time-to-intervention, utility-weighted mRS, rates of conversion to craniotomy, and serious adverse events. See Tables 1 -2 and Figure 1 for details.
Conclusions
Despite a robust improvement in functional outcomes at 30 days, MIS did not improve outcomes through 180 days. MIS was successful in achieving substantial hemorrhage reduction and was not associated with excess peri-procedural mortality or adverse events. Further investigation of MIS in ICH is warranted.
More abstracts on this topic:
Wong Ka-ho, Krothapalli Neeharika, Littig Lauren, Champagne Alison, Majersik Jennifer, Reddy Vivek, De Havenon Adam
A cerebrovascular longitudinal atlas: different rates of morphological change in aneurysm patients associated with hypertension and diabetesChien Aichi, Salamon Noriko, Vinuela Fernando, Szeder Viktor, Colby Geoffrey, Jahan Reza, Boyle Noel, Villablanca Juan, Duckwiler Gary
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.