Final ID: LB39
The Optimal Dosage of ADjunctive Intra-Arterial Tenecteplase following Successful Endovascular Thrombectomy in Patients with Large Vessel Occlusion Acute Ischemic Stroke (DATE) Trail
Xianhua Hou1, Duolao Wang2, Wenjie Zi3, Zhenhua Zhou1 on behalf of DATE investigators, E-mail: exploiter001@126.com.
1.Department of Neurology, Southwest Hospital and The First Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
2.Global Health Trials Unit Liverpool School of Tropical Medicine Liverpool UK
3.Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China.
Trial registration
www.chictr.org.cn (ChiCTR2300073787 and ChiCTR2400080624 ).
Background:
Among patients with acute ischemic stroke (AIS) secondary to large vessel occlusion (LVO) who undergo successful reperfusion following endovascular thrombectomy (EVT), only one-third are disability-free at 90 days, which may be related to the “no-reflow”.
Objective:
To evaluate the promise of efficacy and safety of different dose of adjunctive intra-arterial tenecteplase following successful EVT in patients with LVO.
Methods:
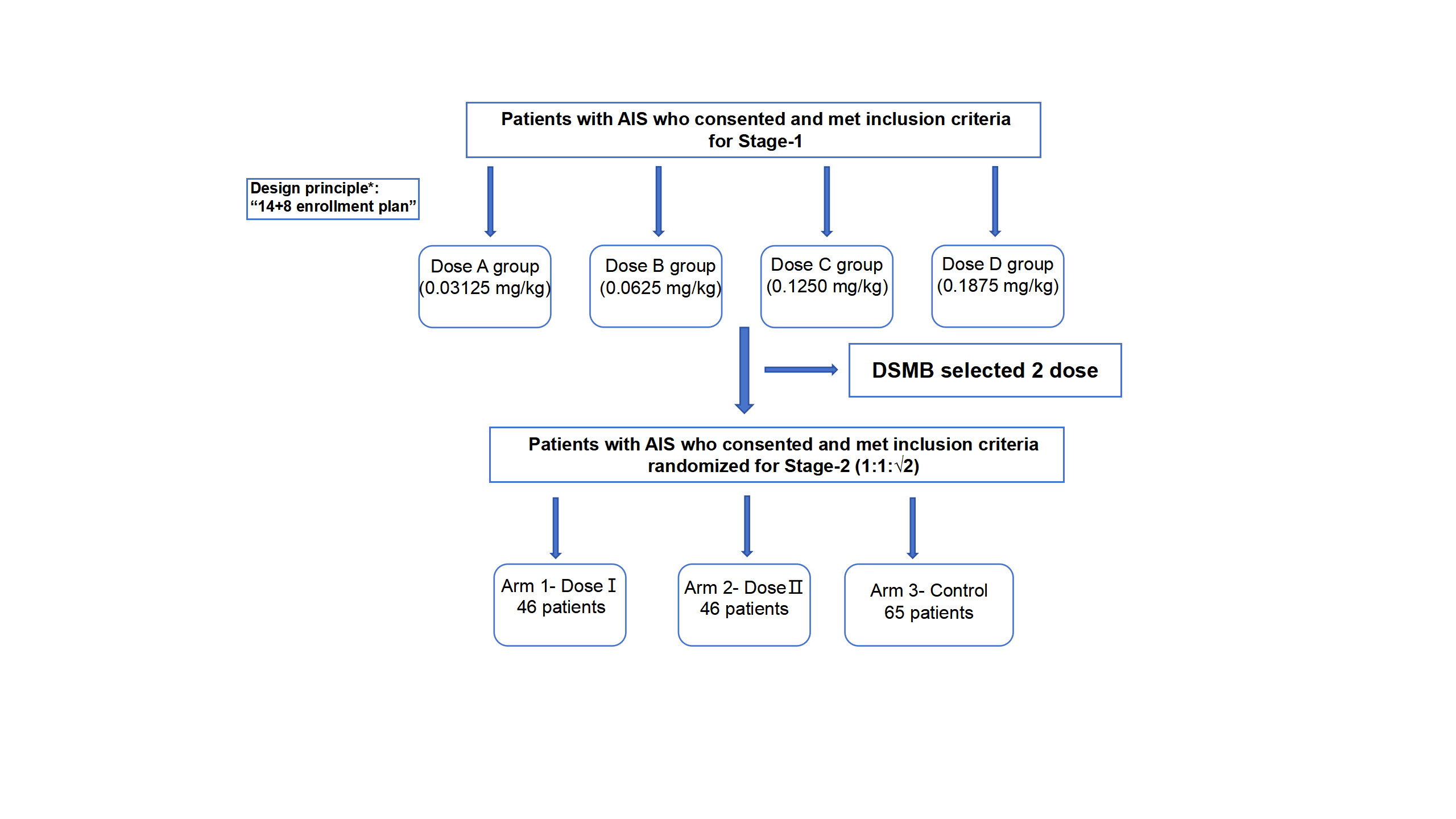
This 2-stage, multicenter, open-label, blind-endpoint, 14+8 dose-escalation (stage-1) and dose-expansion (stage-2) study is conducted to develop initial experience with tenecteplase following successful EVT in patients with LVO within 24h of time last known well. In stage-1 , the dose escalation was conducted in tiers of 14+8 patients, starting at 0.03125 mg/kg(1/8 IV dose), to a planned maximum of 0.1875mg/kg(1/2 IV dose). Based on safety criteria, the best two doses to be administered in stage-2 in which 157 patients will be randomized to three arms.
MAIN OUTCOMES AND MEASURES
The primary outcome was the rate of symptomatic intracranial hemorrhage within 24h in stage-1, and the proportion of patients with modified Rankin scale score of 0 to1 at 90 days in stage-2.
RESULTS:
A total of 48 eligible participants were enrolled in stage-1 from July 2023 to December 2023 from 7 centers in China. A total of 157 eligible participants were enrolled in stage-2 from February 2024 to August 2024 from 23 centers in China. The DATE trial will provide reliable evidence for the optimal dose selection of adjunctive intra-arterial TNK following successful EVT in patients with LVO. Both the results in stages 1 and stage 2 will be presented at the meeting.
More abstracts on this topic:
Xu Xiaohong, Preeti Preeti, Yu Ruoying, Shaykhalishahi Hamed, Zhang Cheng, Shen Chuanbin, Li Bei, Tang Naping, Chang Yan, Xiang Qian, Cui Yimin, Lei Xi, Ni Heyu, Zhu Guangheng, Liu Zhenze, Hu Xudong, Slavkovic Sladjana, Neves Miguel, Ma Wenjing, Xie Huifang
Balloon-mounted versus Self-expanding stents in bail out thrombectomy: impact of the stent design in the RESISTANT registry cohortTomasello Alejandro, Hassan Ameer, Miller Samantha, Zapata-arriaza Elena, De Alboniga-chindurza Asier, Bergui Mauro, Molinaro Stefano, Sousa Joao Andre, Gomes Fábio, Alexandre Andrea, Pedicelli Alessandro, Salcuni Andrea, Hofmeister Jeremy, Machi Paolo, Scarcia Luca, Kalsoum Erwah, Amorim José, Meira Torcato, Ortega-gutierrez Santiago, Rodriguez Aaron, Renieri Leonardo, Capasso Francesco, Kaesmacher Johannes, Gadea Marta, Romano Daniele, Barcena Eduardo, Abdalkader Mohamad, Perry Da Camara Catarina, Yavagal Dileep, Vega Pedro, Ozdemir Atilla Ozcan, Smajda Stanislas, Khalife Jane, Mujanovic Adnan, Biraschi Francesco, Castro Pedro, Siddiqui Adnan, Navia Pedro, Ntoulias Nikolaos, Velo Mariano, Zamarro Joaquin, Zaidat Osama, Sierra-gomez Alicia, Marto Joao Pedro, Geyik Serdar, Requena Manuel, Senadim Songul, Piano Mariangela, Moreu Manuel, Lopez-frias Alfonso
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.