Final ID: LBP61
The safety and feasibility of a pilot clinical trial using combined exercise and neurostimulation for post-stroke pain: the EXERT-Stroke study
Chronic pain after stroke can occur between 10-50% of stroke survivors. Post-stroke pain (PSP) can lead to further complications in a stroke survivor’s recovery. PSP is caused by the stroke itself and produces moderate or severe pain. It can manifest as new onset or worsening of prior headaches.
Methods
EXERT-Stroke was a pilot 2-arm randomized sham-controlled, double-blind trial at a single center over a 30-day intervention period, followed by a month follow-up. Patients were recruited for this study from July 2022 through June 2024 at the Birmingham VA Medical Center. The study protocol was approved by the local institutional review board. The trial was registered with ClinicalTrials.gov (NCT04672044). All potential participants were screened for safety with a graded exercise stress test before randomization. Participants were randomized (1:1) to either active rTMS or sham rTMS. Both arms received the same exercise protocol. The intervention protocol consisted of 10 sessions over a 30-day period of rTMS (sham vs active) + exercise, where rTMS was delivered prior to each exercise session on the same day. RTMS was aimed at the M1 of the contra-lesional hemisphere. Exercise was delivered on a recumbent bicycle using a moderate intensity interval training protocol targeting a participant’s heart rate reserve. Primary outcomes were intervention feasibility (attendance and tolerance) and safety (adverse events).
Results
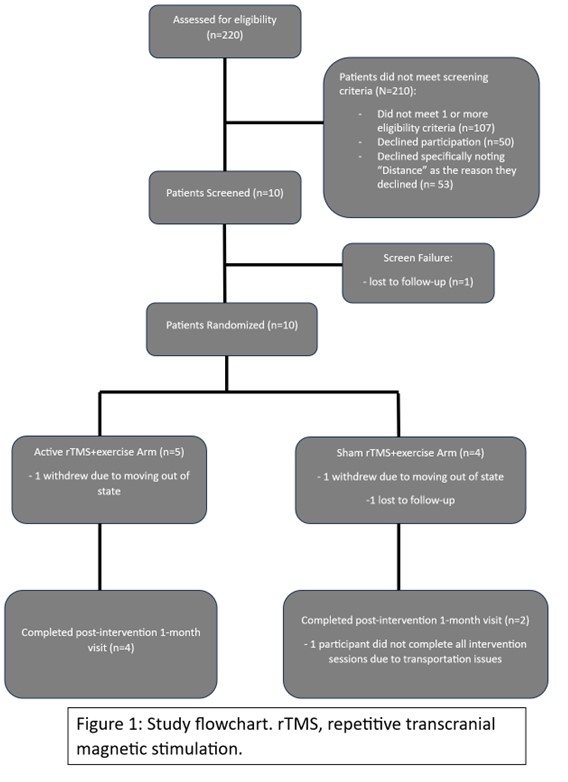
Study flowchart is shown on Figure 1. Of those consented, one participant was a screen failure, and nine participants were randomized. The average age was 62 years old, 22.2% were female, and 44.4% were Black. For feasibility, five (55.5%) participants were randomized to active rTMS and four (44.4%) were randomized to sham rTMS. Four of the five (80%) active rTMS and two of the four (50.0%) of the sham rTMS completed the final assessment, suggesting that there was no association between treatment assignment and likelihood of completing the study. Importantly, there were no serious adverse events. The only adverse event recorded was where one participant reported minor scalp discomfort with active rTMS.
Conclusion
This is the first randomized clinical trial to investigate paired intervention of exercise and rTMS in patients with post-stroke pain and headaches. The trial found that the intervention was both feasible and safe in this population.
More abstracts on this topic:
Yaghmoor Bassam, Hardin Elizabeth, Zink Elizabeth, Pundik Svetlana, Skelly Margaret, Mccabe Jessica, Salameh Ahlam, Bikson Marom, Duncan Kelsey, Mosca Luke, Leonhardt Lisa, Hisel Terri
Cardioprotection by Chemogenetic Stimulation of Hypothalamic Oxytocin Neurons in PrediabetesNilsson Anna, Venter Ian, Carnate Cielo, Kuraoka Kiralee, Escobar Joan, Kay Matthew, Mendelowitz David, Schunke Kathryn
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.