Final ID: OGCTP7
Recombinant Factor VIIa (rFVIIa) for Acute Hemorrhagic Stroke Administered at Earliest Time (FASTEST) Trial
Abstract Body: Objective: The objective of rFVIIa for Acute Hemorrhagic Stroke Administered at Earliest Time (FASTEST) Trial is to establish the first treatment for acute spontaneous ICH within a time window and subgroup of patients that is most likely to benefit. The central hypothesis is that rFVIIa administered within 120 minutes from stroke onset with an identified subgroup of participants most likely to benefit will improve outcomes at 180 days as measured by mRS and decrease ongoing bleeding as compared to standard therapy.
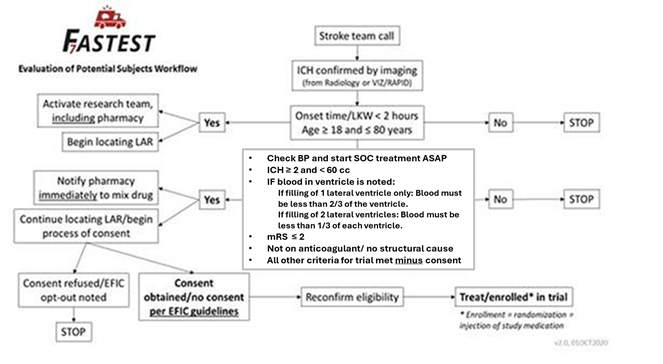
Study Design: Phase III, randomized, double-blind controlled trial of rFVIIa plus best standard therapy vs. placebo and best standard therapy alone. Participants with a volume of ICH ≥ 2 and < 60 cc, filling of < 2/3 of one lateral ventricle of the brain with blood, OR, filling of < 1/3 of both lateral ventricles of the brain with blood, age ≥ 18 and ≤ 80, GCS of ≥ 8, and treated within 120 minutes from stroke onset will be included. To minimize time-to-treatment, the study uses emergency research informed consent procedures (EFIC in the U.S.) and mobile stroke units (MSUs). FASTEST will include approximately 100 hospital sites including approximately 15 MSUs in the NINDS-funded StrokeNet and key global institutions in U.S., Canada, Japan, Germany, Spain, and the U.K. Recruitment of 860 participants over 3½ years is planned.
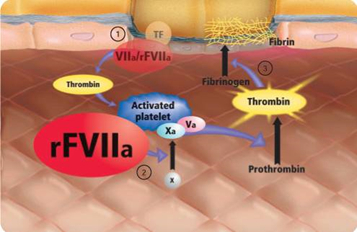
Methodology: Randomization is in a double-blinded fashion to rFVIIa 80 µg/kg dose (maximum 10 mg dose) or placebo. Participants in both arms receive best standard therapy as per published AHA Guidelines for ICH, including a target systolic blood pressure of 140 mm Hg. The primary outcome (ordinal mRS with the following categories: 0-2, 3, and 4-6) is determined at 180 days, with additional assessments at 30 days and 90 days. To measure growth of ICH, all participants have a baseline non-contrast CT of the head and a repeat scan at 24 hours. Centralized volumetric measurements of ICH, IVH, and edema are performed for both time points.
Conclusion: As of October 24th, 2024, 561 participants have been enrolled across 103 active sites, including 52 in the US, 39 OUS and 12 MSU, with an average monthly enrollment rate of 21 over the past year.
Study Design: Phase III, randomized, double-blind controlled trial of rFVIIa plus best standard therapy vs. placebo and best standard therapy alone. Participants with a volume of ICH ≥ 2 and < 60 cc, filling of < 2/3 of one lateral ventricle of the brain with blood, OR, filling of < 1/3 of both lateral ventricles of the brain with blood, age ≥ 18 and ≤ 80, GCS of ≥ 8, and treated within 120 minutes from stroke onset will be included. To minimize time-to-treatment, the study uses emergency research informed consent procedures (EFIC in the U.S.) and mobile stroke units (MSUs). FASTEST will include approximately 100 hospital sites including approximately 15 MSUs in the NINDS-funded StrokeNet and key global institutions in U.S., Canada, Japan, Germany, Spain, and the U.K. Recruitment of 860 participants over 3½ years is planned.
Methodology: Randomization is in a double-blinded fashion to rFVIIa 80 µg/kg dose (maximum 10 mg dose) or placebo. Participants in both arms receive best standard therapy as per published AHA Guidelines for ICH, including a target systolic blood pressure of 140 mm Hg. The primary outcome (ordinal mRS with the following categories: 0-2, 3, and 4-6) is determined at 180 days, with additional assessments at 30 days and 90 days. To measure growth of ICH, all participants have a baseline non-contrast CT of the head and a repeat scan at 24 hours. Centralized volumetric measurements of ICH, IVH, and edema are performed for both time points.
Conclusion: As of October 24th, 2024, 561 participants have been enrolled across 103 active sites, including 52 in the US, 39 OUS and 12 MSU, with an average monthly enrollment rate of 21 over the past year.
More abstracts on this topic:
Association of Post-Stroke Cognitive Impairment with Impaired Glymphatic Function and Neurotoxin Waste Removal in Patients with Intracerebral Hemorrhage
Nariman Nina, Boren Seth, Hasan Khader, Suchting Robert, De Dios Constanza, Sitton Clark, Aronowski Jaroslaw, Savitz Sean, Haque Muhammad
Central nervous system fibroblasts mediate extracellular matrix remodeling in Cerebral Amyloid AngiopathyScott Kiersten, Kyriakopoulos Vasilia, Kim Gab Seok, Lee Juneyoung, Urayama Akihiko
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)