Final ID: 143
Remote Telemedicine Based Enrollment in Acute Stroke Studies at Non-Academic Acute Stroke Ready and Primary Stroke Centers: A Multi-Center Experience of the TIMELESS and FASTEST Trials.
Abstract Body: Introduction:
Clinical trials of acute stroke are often conducted at Comprehensive Stroke Centers (CSC). Limiting enrollment to CSCs can result in slower recruitment, diminished enrollment diversity, and decreased dwell time for recanalization medications. We report the impact of remote telemedicine (TM)-based enrollment at Acute Stroke Ready and Primary Stroke Centers (Non-CSC) in the TIMELESS and FASTEST trials.
Methods:
Both trials were conducted in 2 large health systems with CSC and spoke Non-CSC. Non-CSCs were enabled via video-based TM to remotely screen, consent, randomize and administer study drug locally with a possible transfer to CSC. Follow ups were conducted in person, over the phone or via video TM. Data were retrospectively gathered from the TIMELESS (entire study period) and FASTEST (August 1, 2023-March 31, 2024) studies. Baseline demographics, clinical trial enrollment time metrics, adverse events, missed follow ups and protocol deviations and violations were collected. Data were analyzed with Chi-Square and Kruskall-Wallis.
Results:
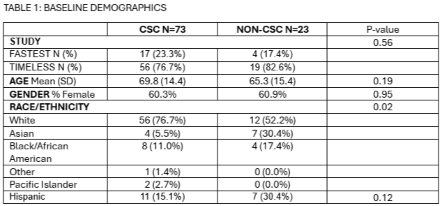
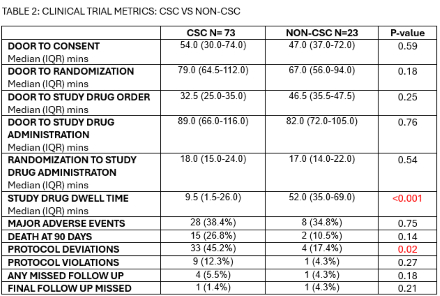
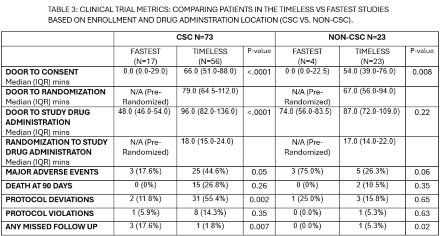
96 patients were enrolled: 75 in TIMELESS and 21 in FASTEST. Twenty-three (24%) were enrolled at Non-CSC:19 (82.6%) in TIMELESS and 4 (17.4%) in FASTEST. There were no differences between CSC vs. Non-CSC enrollments in age, gender, door to consent, door to randomization, door to study drug order placement, door to study drug administration, major adverse events, death at 90 days, protocol violations and missed follow up visits. There was higher racial diversity at Non-CSC enrollments: 30.4% Asian and 17.4% Black vs. CSC: 5.5% Asian and 11.0% Black (p=0.022). There were more protocol deviations identified at the CSC (45.2%) vs. Non-CSC (17.4%) p=0.02. Patients enrolled in TIMELESS at a Non-CSC had a longer median drug dwell time before thrombectomy: 52.0 mins (IQR 35.0-69.0) vs CSC: 9.5 mins (IQR 1.5-26.0) p<.0001. TIMELESS Monthly enrollment increased from 1.24 (CSC) to 1.67 with the addition of Non-CSCs. Similarly, FASTEST monthly enrollment increased from 0.65 to 0.81.
Conclusion:
This is the first study to demonstrate that remote enrollment and study drug administration in acute stroke trials at Non-CSCs are feasible without differences in clinical trial enrollment parameters. It can increase total enrollments, enrich the study populations racial diversity, and increase study drug dwell time which may be important in future studies of extended window thrombolysis and adjunctive recanalization medications.
Clinical trials of acute stroke are often conducted at Comprehensive Stroke Centers (CSC). Limiting enrollment to CSCs can result in slower recruitment, diminished enrollment diversity, and decreased dwell time for recanalization medications. We report the impact of remote telemedicine (TM)-based enrollment at Acute Stroke Ready and Primary Stroke Centers (Non-CSC) in the TIMELESS and FASTEST trials.
Methods:
Both trials were conducted in 2 large health systems with CSC and spoke Non-CSC. Non-CSCs were enabled via video-based TM to remotely screen, consent, randomize and administer study drug locally with a possible transfer to CSC. Follow ups were conducted in person, over the phone or via video TM. Data were retrospectively gathered from the TIMELESS (entire study period) and FASTEST (August 1, 2023-March 31, 2024) studies. Baseline demographics, clinical trial enrollment time metrics, adverse events, missed follow ups and protocol deviations and violations were collected. Data were analyzed with Chi-Square and Kruskall-Wallis.
Results:
96 patients were enrolled: 75 in TIMELESS and 21 in FASTEST. Twenty-three (24%) were enrolled at Non-CSC:19 (82.6%) in TIMELESS and 4 (17.4%) in FASTEST. There were no differences between CSC vs. Non-CSC enrollments in age, gender, door to consent, door to randomization, door to study drug order placement, door to study drug administration, major adverse events, death at 90 days, protocol violations and missed follow up visits. There was higher racial diversity at Non-CSC enrollments: 30.4% Asian and 17.4% Black vs. CSC: 5.5% Asian and 11.0% Black (p=0.022). There were more protocol deviations identified at the CSC (45.2%) vs. Non-CSC (17.4%) p=0.02. Patients enrolled in TIMELESS at a Non-CSC had a longer median drug dwell time before thrombectomy: 52.0 mins (IQR 35.0-69.0) vs CSC: 9.5 mins (IQR 1.5-26.0) p<.0001. TIMELESS Monthly enrollment increased from 1.24 (CSC) to 1.67 with the addition of Non-CSCs. Similarly, FASTEST monthly enrollment increased from 0.65 to 0.81.
Conclusion:
This is the first study to demonstrate that remote enrollment and study drug administration in acute stroke trials at Non-CSCs are feasible without differences in clinical trial enrollment parameters. It can increase total enrollments, enrich the study populations racial diversity, and increase study drug dwell time which may be important in future studies of extended window thrombolysis and adjunctive recanalization medications.
More abstracts on this topic:
A Randomized Comparison of Online Motivational Themes in Cardiovascular Clinical Trial Recruitment
Hussain Zaib, Harry Tamunotonye, Michos Erin, Milller Hailey, Juraschek Stephen, Turkson-ocran Ruth-alma, Lahey Timothy, Feng Yuanyuan, Plante Timothy
Alzheimer’s Disease and Risk of Intracranial HemorrhageZhang Cenai, Bruce Samuel, Navi Babak, Murthy Santosh, Kamel Hooman
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)