Final ID: WP390
Induced Subarachnoid Hemorrhage Leads To Cortical Infarcts In a Mouse Model Of Cerebral Amyloid Angiopathy
Abstract Body: Background: In cerebral amyloid angiopathy (CAA), amyloid-β accumulates within blood vessel walls, leading to hemorrhagic lesions such as convexity subarachnoid hemorrhage (cSAH), microbleeds, and lobar intracerebral hemorrhage. Cortical superficial siderosis (cSS), a chronic result of cSAH, is marked by iron-positive hemosiderin deposits within the superficial cortex and leptomeninges. cSS is associated with an increased risk of cortical microinfarcts, which may contribute to cognitive decline in CAA-related cSS. However, the mechanisms linking cSS to microinfarcts remain unknown. To explore this, we developed a subarachnoid hemorrhage model in the APP23 transgenic (Tg) mouse model of CAA. We hypothesized that mice with subarachnoid hemorrhage would exhibit more infarcts compared to controls, and that infarcts would occur more frequently in Tg mice than in wildtype (WT) littermates.
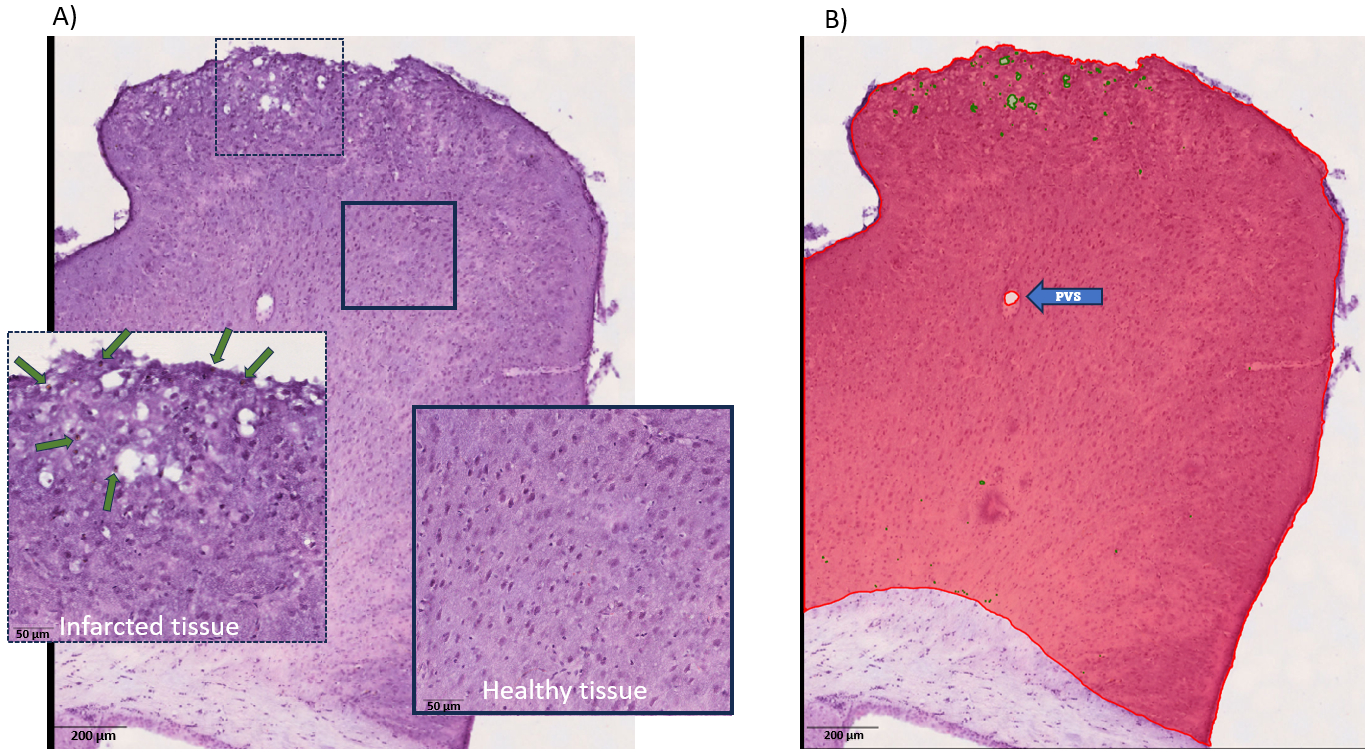
Methods: After skull and dura removal, cranial windows with custom silicone ports were implanted in 11-month-old APP23 Tg and WT mice. One-month post-surgery, 10 µL donor arterial blood or sterile phosphate-buffered saline (PBS) was injected onto the cortex (blood: n=7 WT, 6 Tg; PBS: n=8 WT, 6 Tg). Mice were euthanized one-month post-injection, perfused, and brains were analyzed ex vivo. Serial brain sections were stained with H&E and for iron, and high-resolution images were acquired with a NanoZoomer slide scanner. Infarcts were first qualitatively analyzed by a blinded observer. QuPath, an open-source bioimage analysis software, was then used to quantify infarct volume and iron deposits in cortical tissue. The software was trained with pixel-level annotations to distinguish between healthy brain tissue and ischemic regions as well as to identify iron deposits.
Results: Preliminary analysis revealed hemosiderin deposition in mice that received blood injections, similar to human cSS, and more iron deposits were observed in these mice compared to PBS controls. Qualitative analysis identified more cortical infarcts in regions with hemosiderin than in those without in individual mice. The results of ongoing analyses, including quantitative comparisons between infarcted tissue and iron deposition, will be presented.
Conclusion: This study aims to establish a foundation for quantitatively assessing ischemic tissue and iron deposition in this novel mouse model of cSS, which may be useful for exploring underlying mechanisms and testing future interventions.
Methods: After skull and dura removal, cranial windows with custom silicone ports were implanted in 11-month-old APP23 Tg and WT mice. One-month post-surgery, 10 µL donor arterial blood or sterile phosphate-buffered saline (PBS) was injected onto the cortex (blood: n=7 WT, 6 Tg; PBS: n=8 WT, 6 Tg). Mice were euthanized one-month post-injection, perfused, and brains were analyzed ex vivo. Serial brain sections were stained with H&E and for iron, and high-resolution images were acquired with a NanoZoomer slide scanner. Infarcts were first qualitatively analyzed by a blinded observer. QuPath, an open-source bioimage analysis software, was then used to quantify infarct volume and iron deposits in cortical tissue. The software was trained with pixel-level annotations to distinguish between healthy brain tissue and ischemic regions as well as to identify iron deposits.
Results: Preliminary analysis revealed hemosiderin deposition in mice that received blood injections, similar to human cSS, and more iron deposits were observed in these mice compared to PBS controls. Qualitative analysis identified more cortical infarcts in regions with hemosiderin than in those without in individual mice. The results of ongoing analyses, including quantitative comparisons between infarcted tissue and iron deposition, will be presented.
Conclusion: This study aims to establish a foundation for quantitatively assessing ischemic tissue and iron deposition in this novel mouse model of cSS, which may be useful for exploring underlying mechanisms and testing future interventions.
More abstracts on this topic:
Distinguishing Dystrophic Microglia and Dark Microglia in Ischemic White Matter
Nguyen Hung, Harmon Isabela, Baltan Selva
Cardioprotective Role of Metformin: A Systemic Review and Meta-Analysis on Reducing Reperfusion Injury and Improving Endothelial FunctionNotta Shahnawaz, Magacha Hezborn, Al Akeel Mohannad, Notta Nasir, Jagadish Ashwin, Jbara Manar, Ramu Vijay
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)