Final ID: DP59
Selective Activation of Peritoneal TRPV1-Containing Visceral Afferents Activates Heat Defense and Promotes Sustained Mild Hypothermia and Neuroprotection Following Stroke:
Intro: Mild hypothermia has proved clinically effective at improving outcomes in cardiac arrest patients for decades. Similarly, mild hypothermia has been shown to be neuroprotective in experimental stroke models. Unfortunately, hypothermia protocols in awake subjects are hampered by inconsistent temperature management due to incomplete suppression of cold defense mechanisms (e.g. shiver response). To address the need for better cooling strategies for conscious subjects, we previously demonstrated that pharmacological activation of the heat defense mechanism with TRPV1 agonists can promote sustained mild hypothermia and neuroprotection following stroke. Here, we examined the mechanism by which intraperitoneal delivery of a non-pungent TRPV1 agonist (mixed capsinoid extract; capsiate and dihydrocapsiate) promotes whole body cooling. We hypothesize that capsinoids activate TRPV1-containing visceral afferents in the peritoneal cavity, thereby activating heat defense mechanisms and promoting whole body cooling.
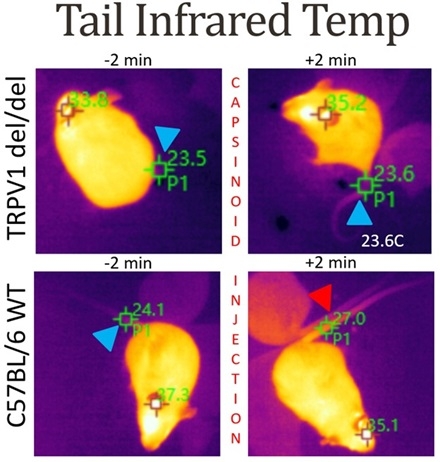
Methods: Adult C57BL6 and TRPV1 knockout (KO) mice of both sexes were given the mixed capsinoids (40 mg/kg, i.p.) or vehicle control. Due to their high lability, capsinoids lack the ability to travel through the body, thereby restricting activity to delivery site. Core body temperature and tail temperature were measured throughout by wireless transponder and FLIR imaging camera, respectively.
Results: Capsinoid administration led to a 1.5 +/- 0.5 C drop in core temperature, reaching its nadir around 20 minutes post-IP injection in C57BL6 males and females (p=0.0057, n=3/4). No temperature drop was evident in the TRPV1 KO mice. Within 2 minutes of capsinoid administration, the tail temperature rose 3.0 +/- 0.4 C (p<0.05, n=3/4), reflecting the activation of heat defense mechanisms (see Figure). Tail temperature did not increase in the TRPV1 KO mice, demonstrating the TRPV1 dependence of this response.
Conclusion: We have previously shown that capsinoid-mediated hypothermia can provide neuroprotection following stroke. We now show that the mechanism of cooling results from selective activation of TRPV1-containing peritoneal visceral afferents and the activation of the heat defense response. Future studies seek to determine the specific nerve pathways linking visceral TRPV1 channel activation and activation of central heat defense mechanisms.
More abstracts on this topic:
Seah Carina, Rivet Dennis, Fraser Justin, Kellner Christopher, Devarajan Alex, Vicari James, Dabney Alan, Baltan Selva, Sohrabji Farida, Pennypacker Keith, Nanda Ashish, Woodward Britton
Cooling the Intestines Offers Superior Protection in the Mouse Stroke ModelLiu Chunli, Park Yujung, Olivas Garcia Yamileck, Villa Jose, Osterli Emily, Chen Yingxin, Hu Bingren
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.