Final ID: TMP80
Outcomes in a national cohort of patients with ischemic stroke who underwent mechanical thrombectomy and decompressive craniectomy were unchanged following extension of the thrombectomy window
In 2015, mechanical thrombectomy (MT) was established as an essential treatment for large-vessel occlusion ischemic stroke (LVO). Since then, trials have expanded the population eligible for MT by demonstrating its benefit in patients with LVO who present later to care and with more extensive infarct. As the eligibility criteria for MT ease, more patients will undergo the procedure who have risk factors for complications – such as malignant edema or hemorrhagic conversion – that require decompressive craniectomy (DC). Though MT and DC in ischemic stroke have been studied independently, the outcomes of patients who undergo both procedures is unknown. We present a case series using a national database of patients who underwent MT and required DC to understand their profile and health outcomes.
Methods
Using the National Inpatient Sample (NIS), an all-payer national healthcare utilization database, patients admitted between the years 2016 and 2021 for ischemic stroke who underwent MT and required DC were identified via ICD-10 codes. Logistic regression was performed to identify patient factors independently associated with DC after MT.
Results
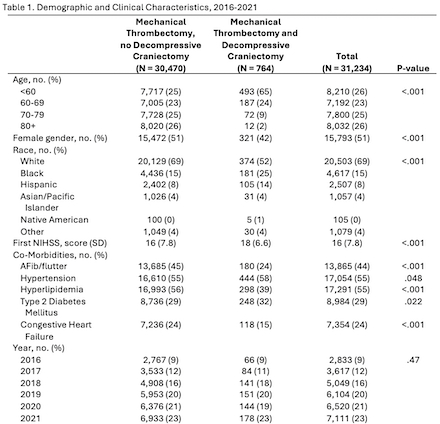
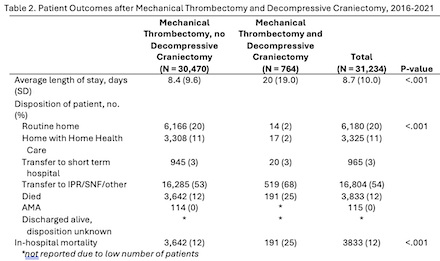
Of the 31,234 patients admitted for LVO who received MT between 2016-2021, 764 (2%) underwent DC (Table 1). Younger age (p < 0.001), non-white race (p < 0.001), a higher NIHSS (p < 0.001), and lower rates of atrial fibrillation were independently associated with DC after MT. Although the number of patients undergoing MT plus DC increased annually, this rise was proportional to the overall number of MT patients. Admissions with MT and DC were nearly 2.5 times longer than those with MT alone (20 days versus 8.4 days, p < .001, Table 2) and associated with increased rates of mortality (25% versus 12%, p < 0.001) and a higher level of care after discharge (p < 0.001).
Conclusion
Rates of DC after MT did not change following extension of the thrombectomy window to 24 hours. DC after MT resulted in a heavy burden of morbidity and mortality, similar to levels previously published for DC after ischemic stroke without MT. In conclusion, our findings suggest that expansion of MT eligibility criteria has not increased the risk for DC and that practice guidelines developed from trials in stroke patients who underwent DC but not MT may also be applicable to patients with DC after MT. Recent large core trials have further expanded the MT population, necessitating continued examination of the relationship between MT and DC.
More abstracts on this topic:
Ang Kevin Royce, Juangco Dan, Hernandez Maria Kim
A First-in-Class Humanized Antibody Fragment Targeting Platelet Glycoprotein Ibα: A Comprehensive Preclinical Study of CA1001 for the Treatment of Acute Ischemic StrokeXu Xiaohong, Preeti Preeti, Yu Ruoying, Shaykhalishahi Hamed, Zhang Cheng, Shen Chuanbin, Li Bei, Tang Naping, Chang Yan, Xiang Qian, Cui Yimin, Lei Xi, Ni Heyu, Zhu Guangheng, Liu Zhenze, Hu Xudong, Slavkovic Sladjana, Neves Miguel, Ma Wenjing, Xie Huifang
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.