Final ID: DP14
Neuroinflammatory mechanisms linking metabolic syndrome with vascular cognitive impairment
Abstract Body: Background: PCSK9, a key regulator of cholesterol metabolism, has been implicated in atherosclerosis. However, its potential role in vascular cognitive impairment and dementia (VCID) remains elusive. We hypothesized that PCSK9 overexpression, exacerbated by a high-fat diet (HFD), would promote neurovascular inflammation and recapitulate VCID pathology.
Methods: C57 mice were stratified into control, PCSK9 overexpression, HFD, and PCSK9+HFD groups. PCSK9 overexpression was induced via AAV injection, and mice were subjected to either a normal diet or HFD for 4 months. Laser speckle contrast imaging was employed to assess CBF response to whisker stimulation. Immunofluorescence analyses were conducted to evaluate neurovascular alterations, encompassing microvascular density, glial activation, neuronal density, arteriosclerosis, and blood-brain barrier integrity. Light sheet microscopy was utilized to provide ultra-high resolution 3D imaging of the cerebrovascular architecture. Cognitive function and exploratory behavior were investigated using novel object recognition and open field tests.
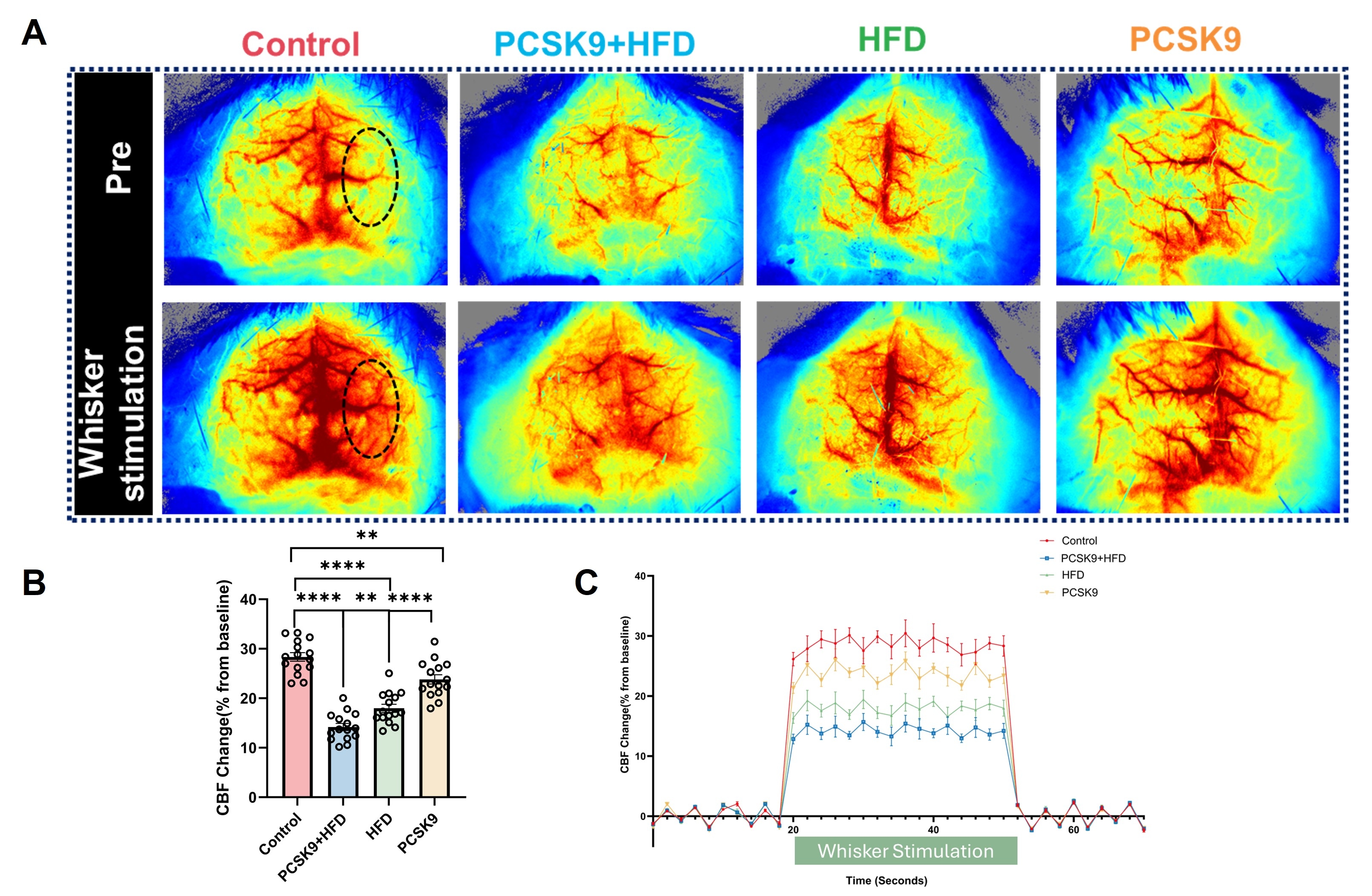
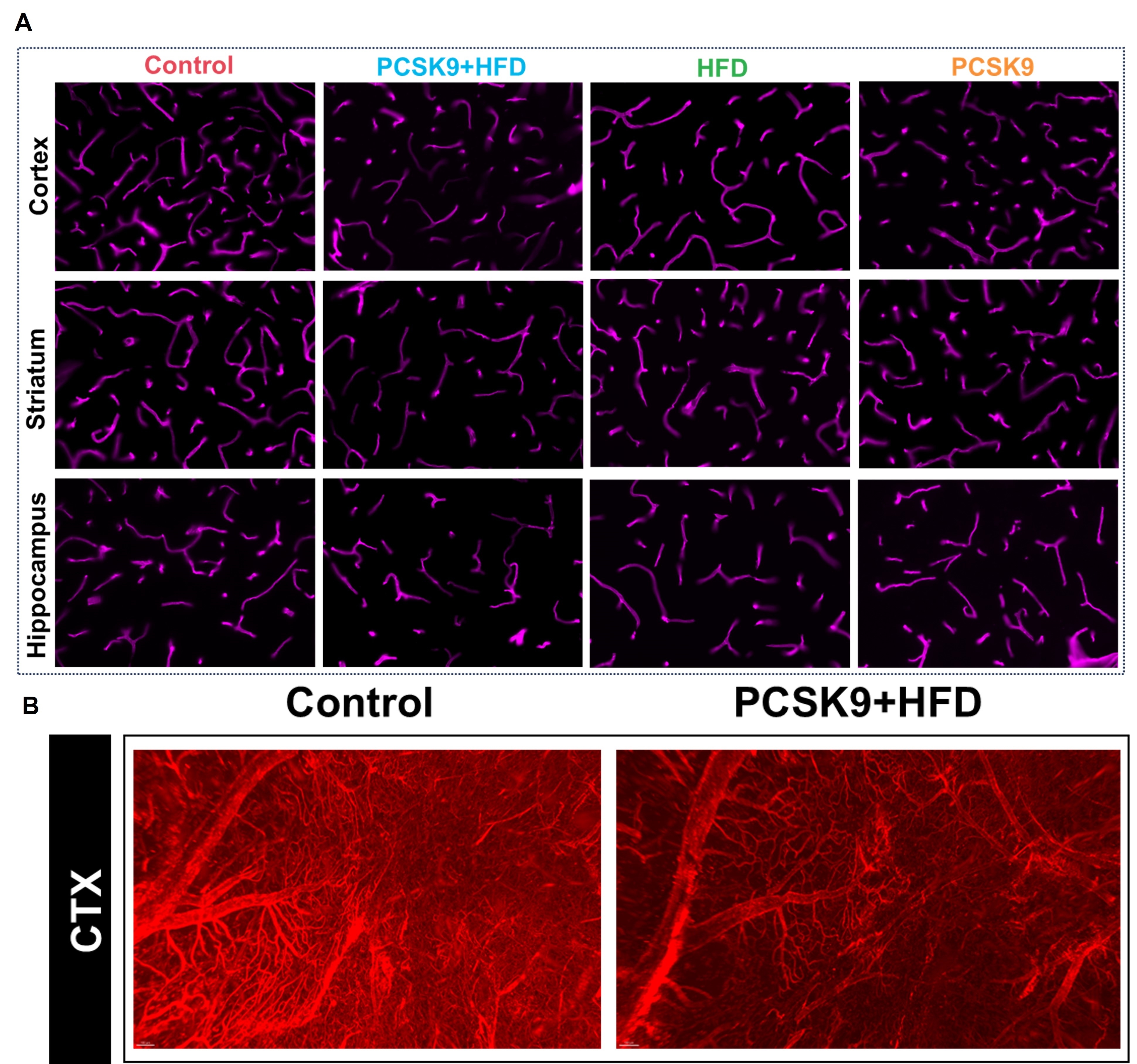
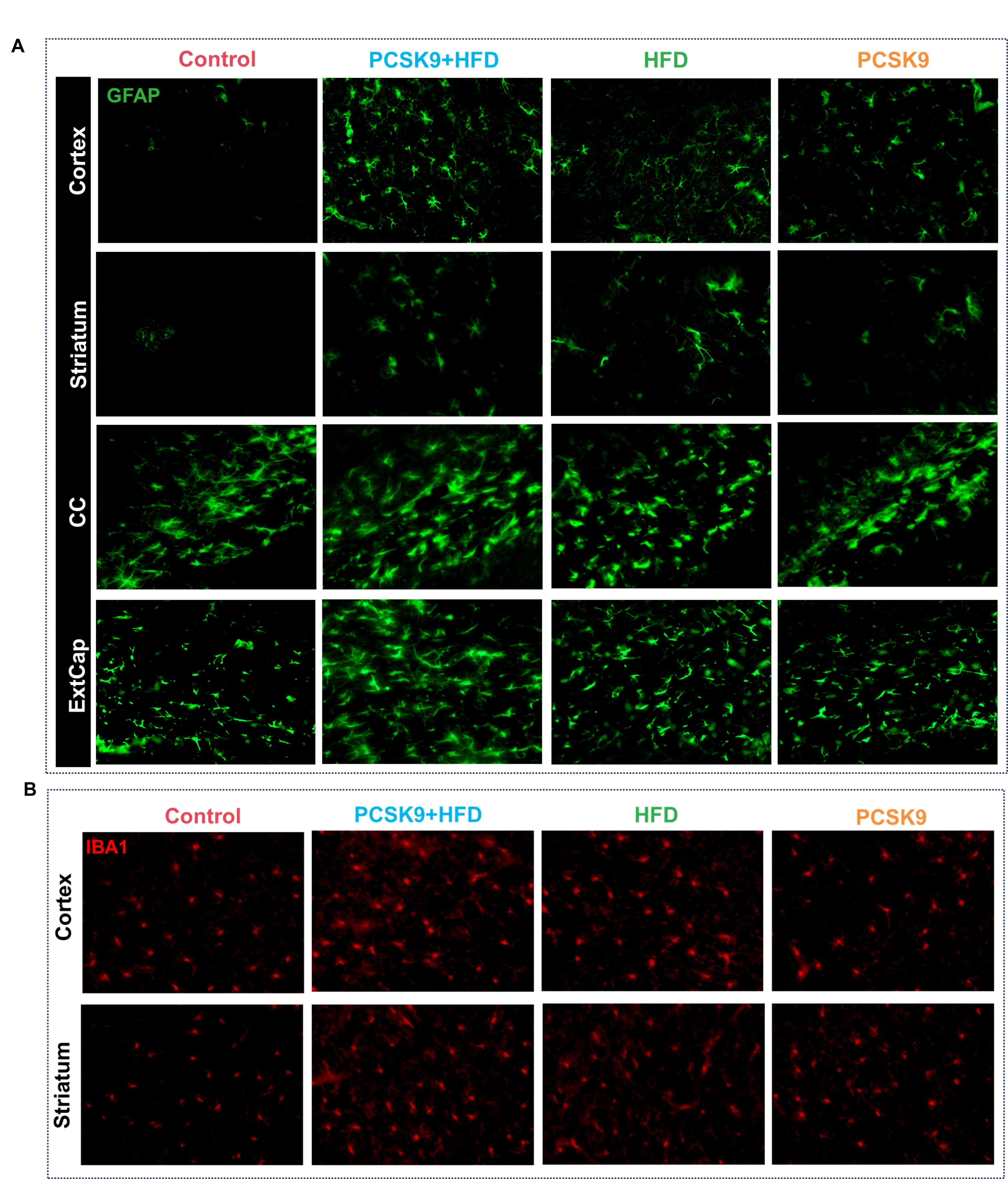
Results: PCSK9+HFD mice exhibited significantly compromised neurovascular coupling, as evidenced by attenuated CBF response to whisker stimulation. Immunofluorescence revealed profound neurovascular alterations, including markedly reduced microvascular density, exacerbated astrogliosis and microglial activation, diminished neuronal density, heightened arteriosclerosis and inflammation, and severe blood-brain barrier disruption. These changes were most pronounced in the PCSK9+HFD group, followed by the HFD and PCSK9 groups, highlighting the synergistic detrimental effects of PCSK9 overexpression and HFD. Notably, light sheet fluorescence microscopy (LSFM) unveiled a dramatically sparser and less complex 3D cerebrovascular network in PCSK9+HFD mice. Moreover, PCSK9+HFD mice displayed the most severe neurocognitive changes, as shown by impaired recognition memory and reduced exploration.
Conclusions: This study demonstrates that metabolic syndrome induced by PCSK9 overexpression and HFD synergistically converge upon neuroinflammatory mediators to recapitulate VCID pathology. Microglial and astrocytic activation in PCSK9 overexpression and HFD is strongly associated with altered cerebral blood flow regulation. Our findings provide a preclinical platform for discovery of new therapeutic approaches for neuroinflammatory and cerebrovascular alterations associated with VCID pathology.
Methods: C57 mice were stratified into control, PCSK9 overexpression, HFD, and PCSK9+HFD groups. PCSK9 overexpression was induced via AAV injection, and mice were subjected to either a normal diet or HFD for 4 months. Laser speckle contrast imaging was employed to assess CBF response to whisker stimulation. Immunofluorescence analyses were conducted to evaluate neurovascular alterations, encompassing microvascular density, glial activation, neuronal density, arteriosclerosis, and blood-brain barrier integrity. Light sheet microscopy was utilized to provide ultra-high resolution 3D imaging of the cerebrovascular architecture. Cognitive function and exploratory behavior were investigated using novel object recognition and open field tests.
Results: PCSK9+HFD mice exhibited significantly compromised neurovascular coupling, as evidenced by attenuated CBF response to whisker stimulation. Immunofluorescence revealed profound neurovascular alterations, including markedly reduced microvascular density, exacerbated astrogliosis and microglial activation, diminished neuronal density, heightened arteriosclerosis and inflammation, and severe blood-brain barrier disruption. These changes were most pronounced in the PCSK9+HFD group, followed by the HFD and PCSK9 groups, highlighting the synergistic detrimental effects of PCSK9 overexpression and HFD. Notably, light sheet fluorescence microscopy (LSFM) unveiled a dramatically sparser and less complex 3D cerebrovascular network in PCSK9+HFD mice. Moreover, PCSK9+HFD mice displayed the most severe neurocognitive changes, as shown by impaired recognition memory and reduced exploration.
Conclusions: This study demonstrates that metabolic syndrome induced by PCSK9 overexpression and HFD synergistically converge upon neuroinflammatory mediators to recapitulate VCID pathology. Microglial and astrocytic activation in PCSK9 overexpression and HFD is strongly associated with altered cerebral blood flow regulation. Our findings provide a preclinical platform for discovery of new therapeutic approaches for neuroinflammatory and cerebrovascular alterations associated with VCID pathology.
More abstracts on this topic:
Cardiovascular risk factors for dementia: Causal estimates on individual-level data
Luo Jiao, Juul Rasmussen Ida, Qvist Thomassen Jesper, Frikke-schmidt Ruth
Chronic cerebral hypoperfusion impairs brain iron metabolism in aged miceMonga Sheelu, Moruno-manchon Jose
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)