Final ID: TP21
Efficacy and Safety of Sacubitril/Valsartan in Patients with Acute Ischemic Stroke and Hypertension within 24-72 hours
Methods:Between November 2022 and May 2024, patients with AIS within 24-72h of symptom onset and BP≥140/90 mmHg were enrolled and randomly assigned to receive Sacubitril/Valsartan (100-200mg qd) or other antihypertensive medications determined by clinical physicians as the ratio of 1:1. Randomized antihypertensive treatment strategy was required to complied for 14 days, and allowed to be changed after day 14 if the BP control was not satisfied. All enrolled patients received other early medical managements based on the AIS guideline. The primary outcome was the proportion of participants who had a modified Rankin scale(mRS) score of 0-1 at 90 days. The secondary outcome was the rate of target BP control (mean BP<140/90mmHg) at day 14, the mRS distribution at 90days. The safety outcome was the rate of hypotension (<100/60mmHg at any measurement within 14 days).
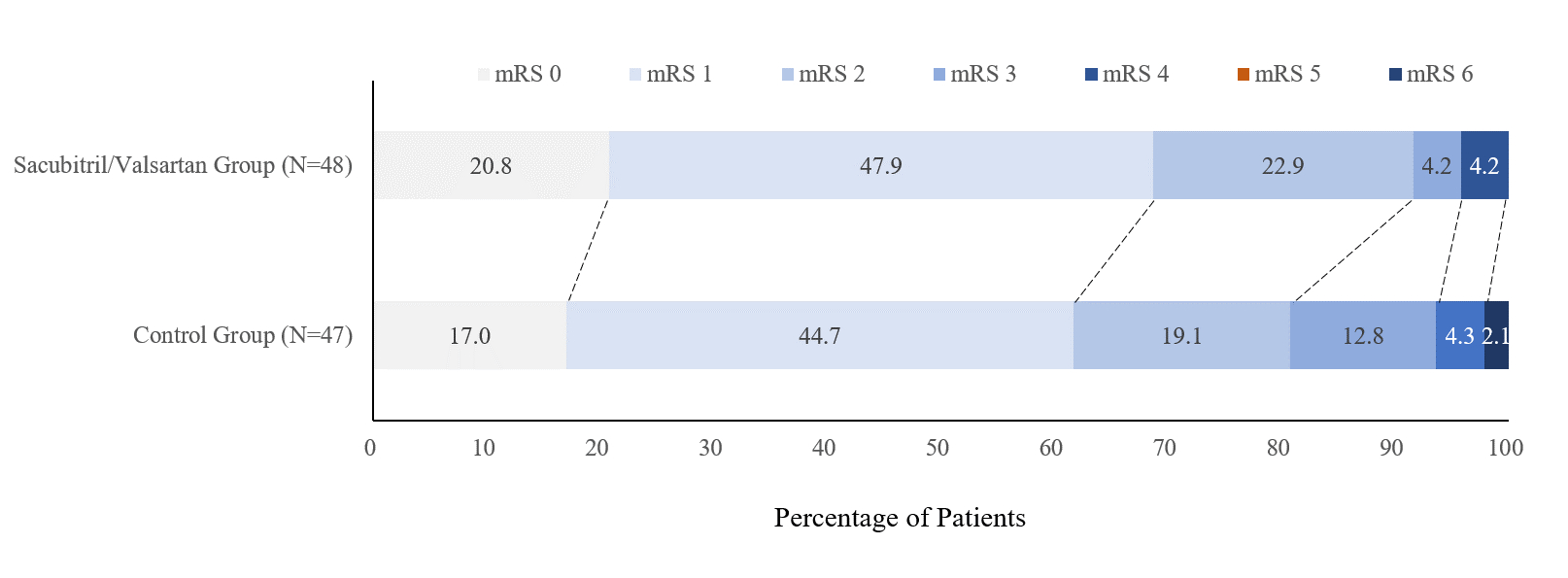
Results: Forty-eight patients were assigned to the Sacubitril/Valsartan group and forty-seven were assigned to the group of other antihypertensive agents, with mean time from symptom onset to randomization was 60.0±11.3 hours and 57.0±14.8 hours, respectively. Mean systolic BP was reduced from 162mmHg to 136mmHg in the Sacubitril/Valsartan group and from 162mmHg to 137mmHg in the control group after 14 days. Baseline NIHSS in two groups was 3.5±1.5 and 3.7±2.3, respectively. The proportion of mRS 0-1 at day 90 did not differ between two treatment groups. The rate of BP target was not significantly different between two groups at day 14. Sacubitril/Valsartan group showed a trend of lower mRS than the control group, though no significant difference was found (1.2±0.9 vs 1.5±1.2, p=0.22). No hypotension occurred in either group.
Conclusions: For mild AIS patients within 24-72 hours of symptom onset, Sacubitril/Valsartan showed similar efficiency and safety in reducing high blood pressure to other antihypertensive agents. Although it did not increase the proportion of mRS0-1 at day 90, a trend of lower mRS in the Sacubitril/Valsartan group was demonstrated. Multi-center RCT studies including AIS patients with high NIHSS score are warranted to further confirm these results.
More abstracts on this topic:
Xu Xiaohong, Preeti Preeti, Yu Ruoying, Shaykhalishahi Hamed, Zhang Cheng, Shen Chuanbin, Li Bei, Tang Naping, Chang Yan, Xiang Qian, Cui Yimin, Lei Xi, Ni Heyu, Zhu Guangheng, Liu Zhenze, Hu Xudong, Slavkovic Sladjana, Neves Miguel, Ma Wenjing, Xie Huifang
3CPR Best Abstract Award: The pathogenic role of ADAMTS13 deficiency in Chronic Thromboembolic Pulmonary HypertensionWu Zhijian, Zheng X. Long, Zheng Liang
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.