Final ID: WP118
Lost to Follow-Up in Randomized Clinical Trials on Longer-Term Patient Management Following Stroke
Although long-term management following a stroke is crucial, many patients do not adhere to follow-up appointments, which pose a risk to the integrity of clinical trials. This systematic survey aimed to identify factors and potential impacts linked to lost to follow-up (LTFU) in stroke patients participating in long-term management trials, to enhance trial quality. Understanding LTFU is essential for informing patients, clinicians, and researchers for both clinical and research purposes.
Methods
An information specialist completed a comprehensive search of available data sources, including studies published up to June 15, 2024. Eligible articles included randomized trials that involved multimodal post-stroke care programs, initiated within one year after the stroke, with specified and assessed follow-up outcomes. We collected data on general trial characteristics and methodological characteristics for each study. Multiple linear regression analyses were conducted to identify factors associated with LTFU. Additionally, we evaluated the relative risk of several assumptions about the outcomes of participants LTFU on the estimate of effect for the significant binary primary outcome.
Results
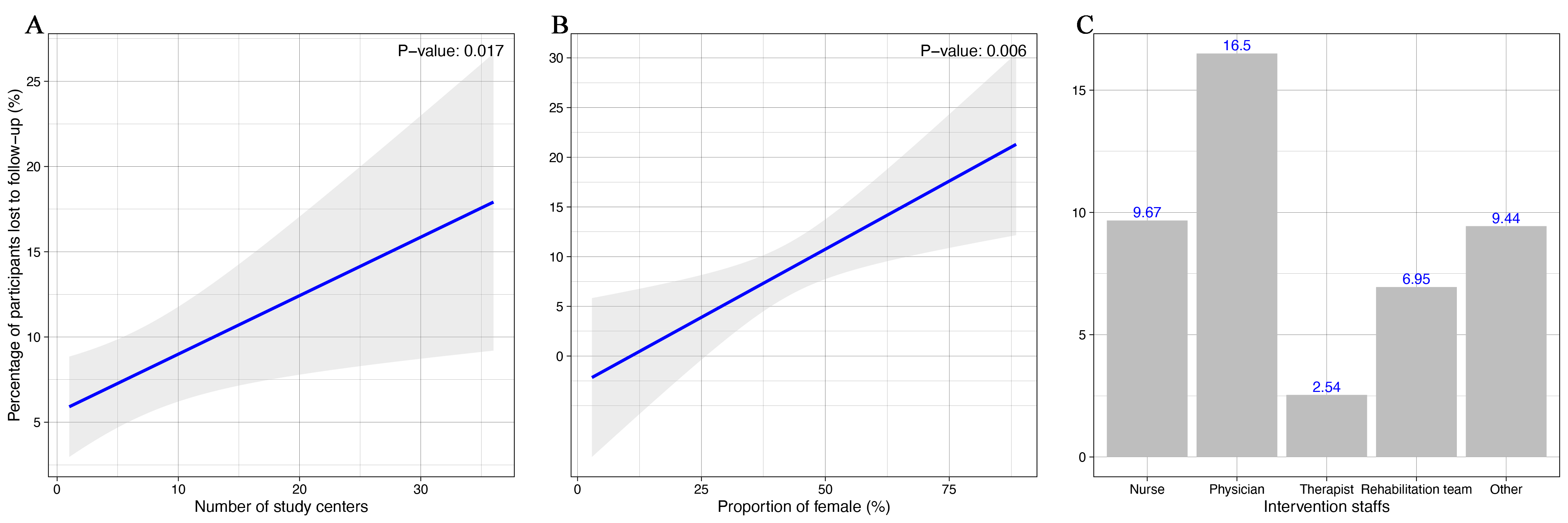
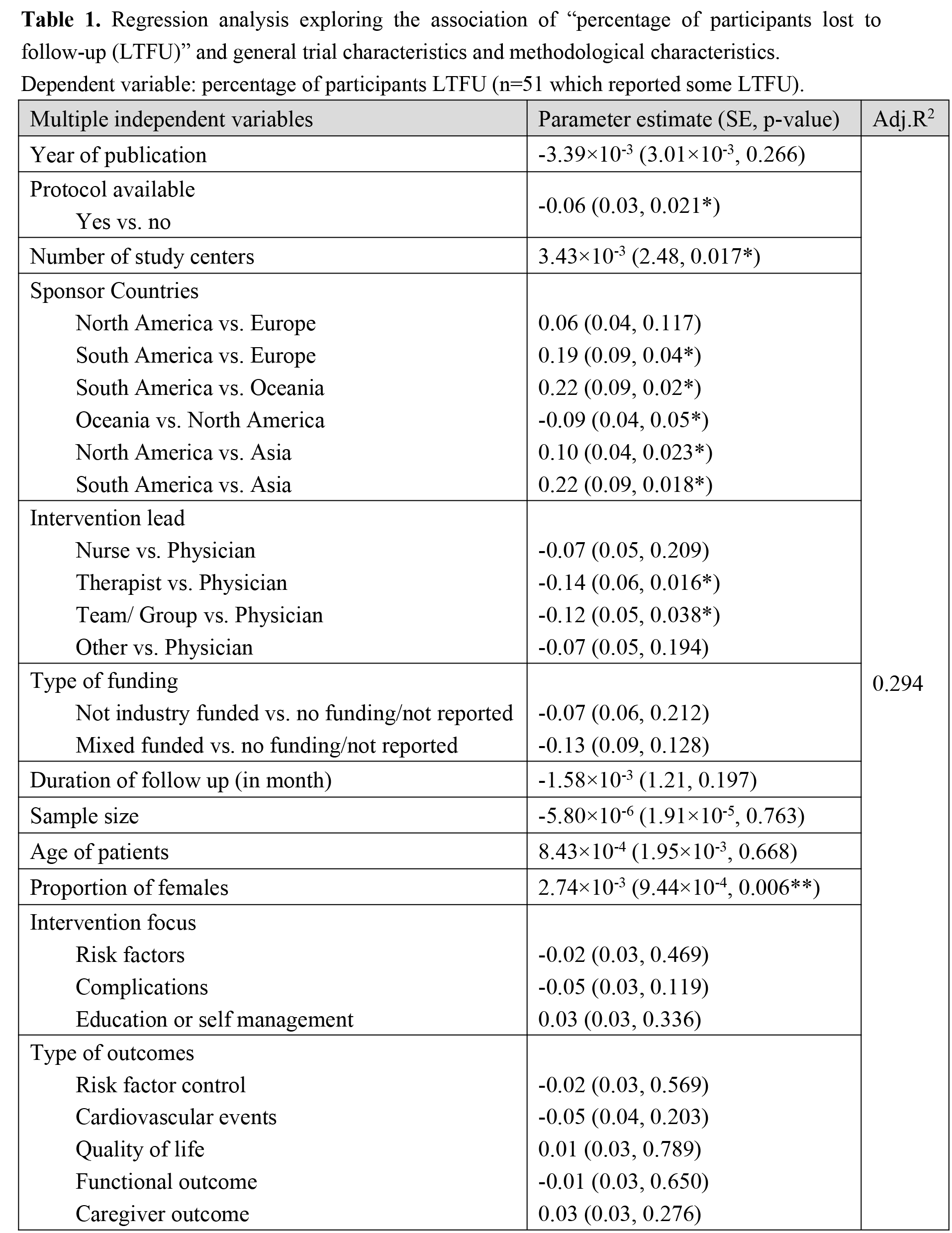
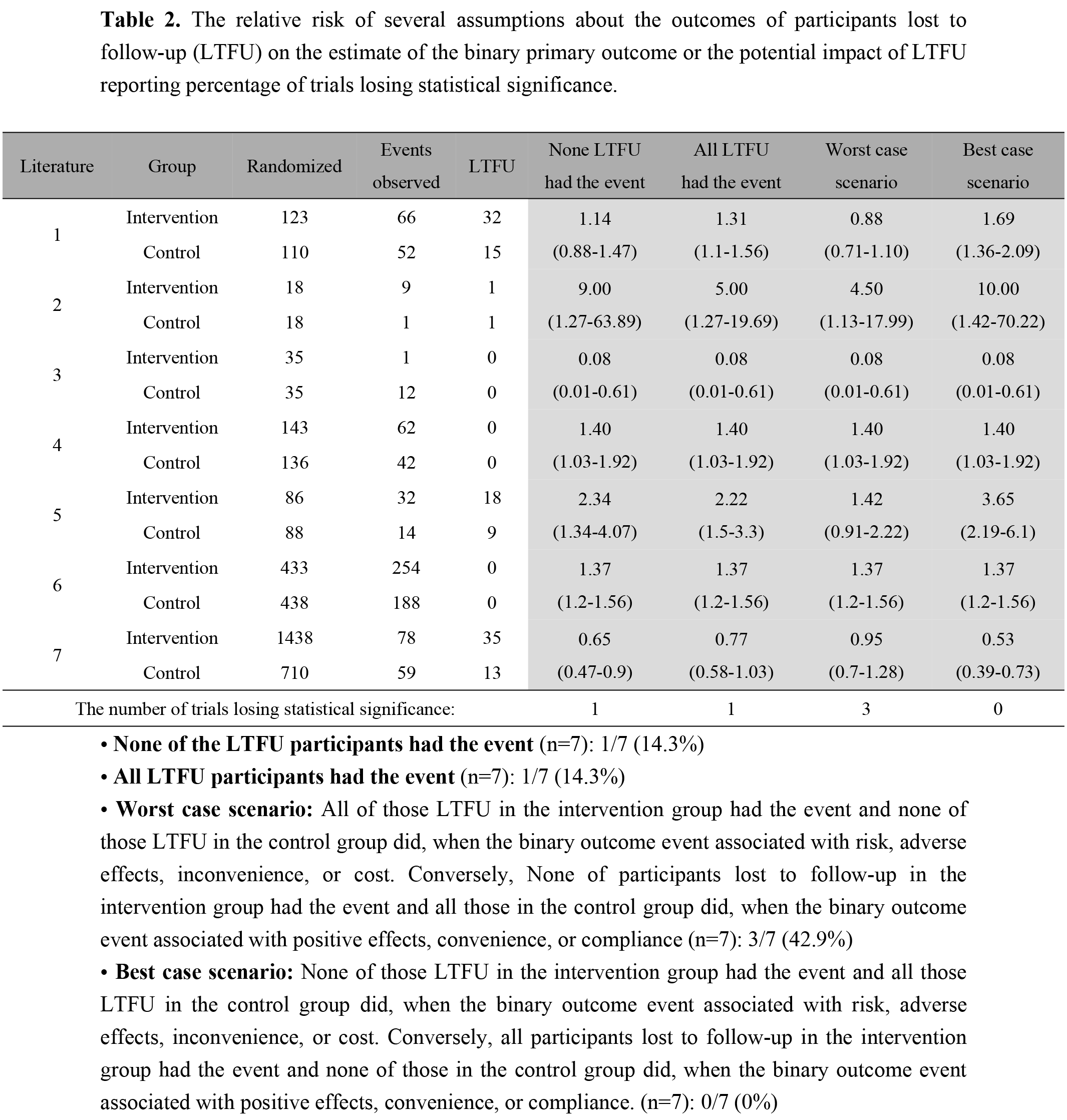
Of the 57 eligible reports identified, 6 (10.5%) did not specify whether LTFU occurred. The duration of follow-up ranged from 1 to 60 months. The median proportion of subjects LTFU was 7.9% (interquartile range, 0–12.9%). Factors of LTFU increase on regression analysis were more study centers (beta=0.003 per center, P=0.017), higher proportion of females (beta=0.027 per percentage of 10, P=0.006), and non-protocol available (beta=-0.06, P=0.021) (Table 1, Fig.1). Patients were also more likely not to be lost if their providers were therapists (beta=-0.14, P=0.016) or rehabilitation team (beta=-0.12, P=0.038) compared to physician alone. In the trials that provided relevant data, results of 14.3% of trials were no longer significant if we assumed no participants LTFU had the event of interest, and 42.9% of trials were no longer significant if we assumed a worst-case scenario (Table 2).
Conclusion
Overall, nearly one-tenth of contemporary stroke trials on longer-term patient management still did not report LTFU. Identified modifiable risk factors may provide targets to improve the continuity of stroke management within these trial settings. Neurologists should pay close attention to how the participants are managed which may change the direction of the entire study.
More abstracts on this topic:
Murakami Tatsuaki
Impact of Anemia on the Prediction of Galectin-3 for Cause-specific Mortality in Patients With Suspected or Known Coronary Artery Disease: The ANOX StudyWada Hiromichi, Takenaka Takashi, Morita Yukiko, Nakamura Toshihiro, Fujimoto Kazuteru, Matsubara Hiromi, Kato Toru, Unoki Takashi, Takagi Daisuke, Wada Kyohma, Wada Miyaka, Suzuki Masahiro, Nakayama Takumi, Maeda Yuka, Masunaga Nobutoyo, Ishii Mitsuru, Kotani Kazuhiko, Abe Mitsuru, Akao Masaharu, Hasegawa Koji, Matsuda Morihiro, Ajiro Yoichi, Shinozaki Tsuyoshi, Sakagami Satoru, Yonezawa Kazuya, Shimizu Masatoshi, Funada Junichi
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.