Final ID: TMP31
Distinguishing Intracerebral Hemorrhage from Acute Cerebral Ischemia in the Prehospital Setting: Development and Validation of the California Acute stroke Subtype PRehospital (CASPR) Scale
Abstract Body: Background: A prehospital, paramedic-administered scale to distinguish intracerebral hemorrhage (ICH) from acute cerebral ischemia (ACI) could improve routing to appropriate centers, enrich field randomized trials with targeted subtype patients, and potentially guide prehospital clinical treatment such as hyperacute blood pressure (BP) lowering. We aimed to create a quickly administered prehospital scale from prospectively performed field assessments.
Methods: Two scales were created from NIH Field Administration of Stroke Therapy Magnesium (FAST-MAG) trial data, using logistic regression model with backward stepwise variable selection and retention criterion of p<0.1. Scale one—CASPR-DB (Data-Based)—assessed 26 candidate variables available in routine prehospital care in 1675 patients (77.3% ACI, 22.7% ICH), who were randomly assigned to training (n=1114) or validation (n=561) sets. Scale two—CASPR-CS (Clinical Symptoms)—came from a subset of patients (n=467) in whom three additional clinical symptoms elicited in the field were also candidate items—headache, nausea, and progressing deficit.
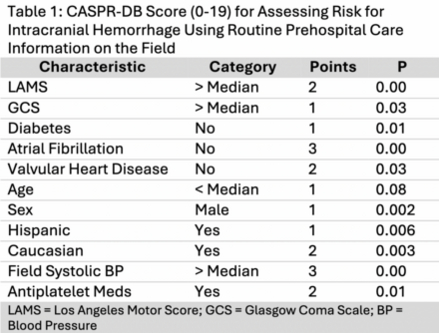
Results: For the CASPR-DB scale, the logistic regression model selected 10 of the 26 candidate variables in the training population. Factors associated with ICH were higher Los Angeles Motor Scale (LAMS) score, higher Glasgow Coma Score (GCS), higher systolic BP, Hispanic and Caucasian race-ethnicity, male sex, use of anti-platelet agents, decreased age, and absence of diabetes, atrial fibrillation, or valvular heart disease. Each was given a point value between 1-3, yielding a 19-point scale (Table 1).
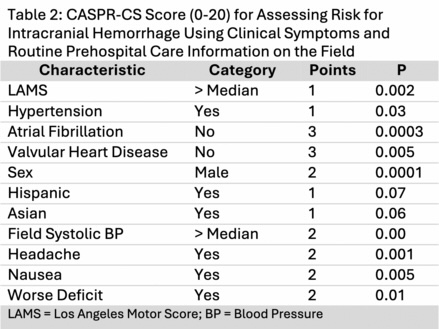
For the CASPR-CS scale, the logistic regression model selected 10 of the 29 candidate variables. All three symptom items—headache, nausea, and progressing deficit—were associated with ICH, along with 7 data-based variables. Each was given a point value between 1-3, yielding a 20-point scale (Table 2).
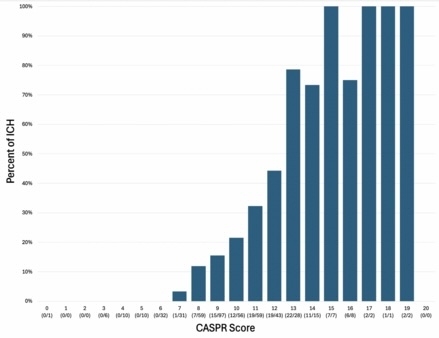
CASPR-DB performance in training was sensitivity 64.4%, specificity 78.6%, accuracy 71.5%, c=0.774 and in validation was sensitivity 69.3%, specificity 61.7%, accuracy 71.5%, c=0.710. CASPR-CS performance was sensitivity 71.8%, specificity 77.8%, accuracy 74.8%, c=0.830. Figure 1 shows the proportions of ICH patients at each CASPR-CS score level.
Conclusions: The CASPR-DB scale shows good and CASPR-CS scale very good performance in distinguishing ICH vs ACI in prehospital setting. These readily performable scales could improve prehospital routing and treatment of ICH patients.
Methods: Two scales were created from NIH Field Administration of Stroke Therapy Magnesium (FAST-MAG) trial data, using logistic regression model with backward stepwise variable selection and retention criterion of p<0.1. Scale one—CASPR-DB (Data-Based)—assessed 26 candidate variables available in routine prehospital care in 1675 patients (77.3% ACI, 22.7% ICH), who were randomly assigned to training (n=1114) or validation (n=561) sets. Scale two—CASPR-CS (Clinical Symptoms)—came from a subset of patients (n=467) in whom three additional clinical symptoms elicited in the field were also candidate items—headache, nausea, and progressing deficit.
Results: For the CASPR-DB scale, the logistic regression model selected 10 of the 26 candidate variables in the training population. Factors associated with ICH were higher Los Angeles Motor Scale (LAMS) score, higher Glasgow Coma Score (GCS), higher systolic BP, Hispanic and Caucasian race-ethnicity, male sex, use of anti-platelet agents, decreased age, and absence of diabetes, atrial fibrillation, or valvular heart disease. Each was given a point value between 1-3, yielding a 19-point scale (Table 1).
For the CASPR-CS scale, the logistic regression model selected 10 of the 29 candidate variables. All three symptom items—headache, nausea, and progressing deficit—were associated with ICH, along with 7 data-based variables. Each was given a point value between 1-3, yielding a 20-point scale (Table 2).
CASPR-DB performance in training was sensitivity 64.4%, specificity 78.6%, accuracy 71.5%, c=0.774 and in validation was sensitivity 69.3%, specificity 61.7%, accuracy 71.5%, c=0.710. CASPR-CS performance was sensitivity 71.8%, specificity 77.8%, accuracy 74.8%, c=0.830. Figure 1 shows the proportions of ICH patients at each CASPR-CS score level.
Conclusions: The CASPR-DB scale shows good and CASPR-CS scale very good performance in distinguishing ICH vs ACI in prehospital setting. These readily performable scales could improve prehospital routing and treatment of ICH patients.
More abstracts on this topic:
Central nervous system fibroblasts mediate extracellular matrix remodeling in Cerebral Amyloid Angiopathy
Scott Kiersten, Kyriakopoulos Vasilia, Kim Gab Seok, Lee Juneyoung, Urayama Akihiko
Airway Opening Index is Associated with Return of Spontaneous Circulation in Swine and Humans with Cardiac ArrestBhandari Shiv, Coult Jason, Sharpe Zachary, Rea Thomas, Neumar Robert, Hsu Cindy, Counts Catherine, Sayre Michael, Johnson Nicholas
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)