Final ID: WP44
Homocysteine mediates the impact of PFO shunt on vascular cognitive impairment
Abstract Body: Background
Patent foramen ovale (PFO) is an independent risk factor for neurovascular injury such as stroke. We previously found that large PFO shunt is associated with increased long-term risk of vascular dementia. However, the mechanism underlying this association remains poorly understood. Our previous research revealed that PFO shunt enables the accumulation of homocysteine in circulation (Deng, Neurology 2021). It is thus possible that homocysteine may mediate the effect of PFO shunt on cognitive decline. This study aims to explore the relationship among PFO shunt, total homocysteine (tHcy) levels and the risk of vascular cognitive impairment and dementia (VCID).
Method
A total of 1282 PFO patients were prospectively recruited and followed for over 11 years post their PFO closure in accordance with IRB protocol. All the patients were cognitively normal at the time of PFO diagnosis. Residual shunt post PFO closure was assessed using transthoracic echocardiogram (TTE) with saline contrast. Cognitive function was evaluated using Montreal Cognitive Assessment (MoCA). tHcy levels in peripheral venous blood was measured using mass spectrometry.
Result
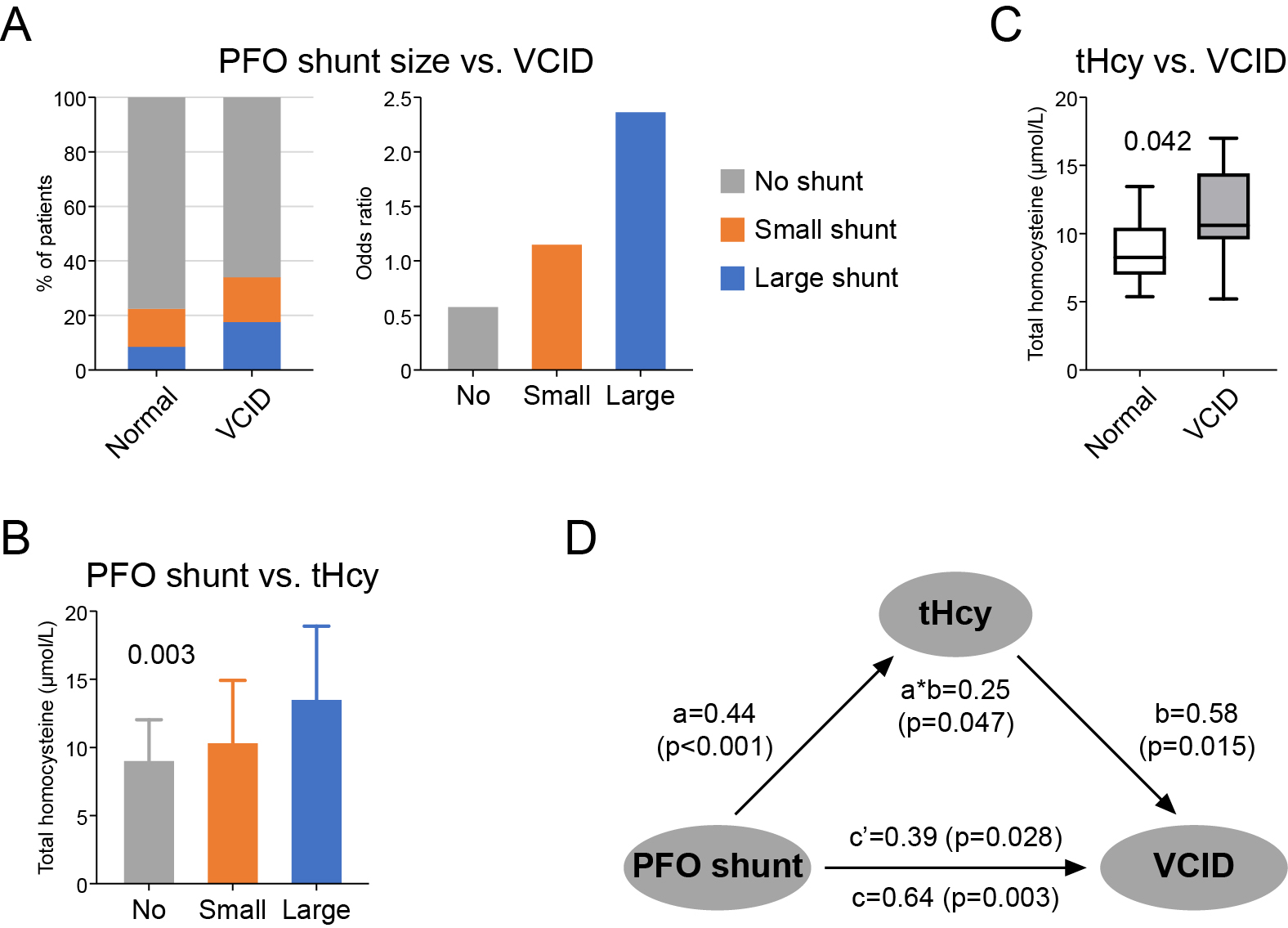
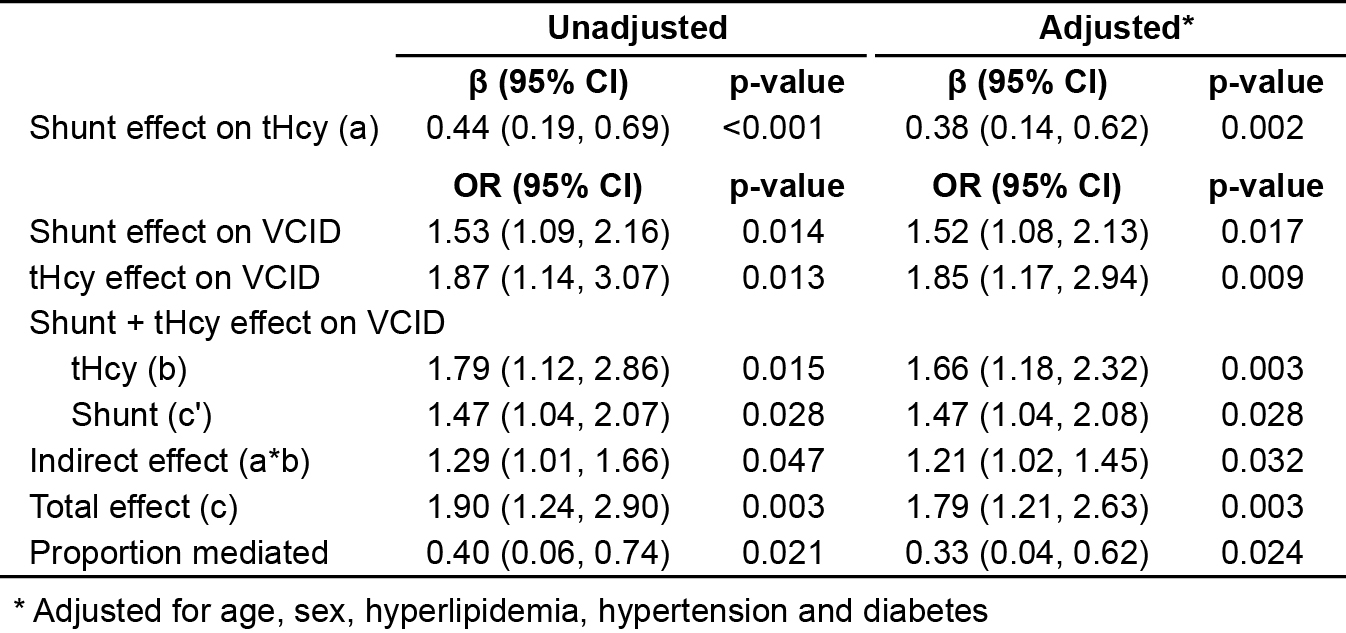
Among the patients, 67 (5.2%) developed VCID as measured by MoCA < 26 and NINDS AIREN criteria. The risk of VCID was significantly high in patients with residual shunt, particularly those with large shunt size (no shunt: 5.0%; small shunt: 6.4%; large shunt: 11.5%), suggesting a dose effect of PFO shunt (OR: 1.53; 95% CI: 1.09 ~ 2.16; p = 0.014) (Figure 1A, Table 1). Moreover, residual PFO shunt also contributed to tHcy elevation in circulation (β: 0.44; 95% CI: 0.19 ~ 0.69; p < 0.001) (Figure 1B, Table 1), and high tHcy levels were linked to increased risk of VCID (OR: 1.87; 95% CI: 1.14 ~ 3.07; p = 0.013) (Figure 1C, Table 1). Further mediation analyses revealed a significant indirect effect of PFO shunt on VCID through tHcy (OR: 1.29; 95% CI: 1.01 ~ 1.66; p = 0.047) (Figure 1D, Table 1). tHcy mediated 40% (95% CI: 6% ~ 74%; p = 0.021) of the total association between PFO shunt and VCID risk (Figure 1D, Table 1). These findings remained robust after adjusting for other VCID risk factors, such as age, diabetes, hyperlipidemia and hypertension (Table 1).
Conclusion
Our study suggested that that PFO shunt may contribute to the development of VCID by promoting circulatory tHcy elevation. Further mechanistic investigation of neurovascular injury and cognitive decline associated with PFO shunt is ongoing.
Patent foramen ovale (PFO) is an independent risk factor for neurovascular injury such as stroke. We previously found that large PFO shunt is associated with increased long-term risk of vascular dementia. However, the mechanism underlying this association remains poorly understood. Our previous research revealed that PFO shunt enables the accumulation of homocysteine in circulation (Deng, Neurology 2021). It is thus possible that homocysteine may mediate the effect of PFO shunt on cognitive decline. This study aims to explore the relationship among PFO shunt, total homocysteine (tHcy) levels and the risk of vascular cognitive impairment and dementia (VCID).
Method
A total of 1282 PFO patients were prospectively recruited and followed for over 11 years post their PFO closure in accordance with IRB protocol. All the patients were cognitively normal at the time of PFO diagnosis. Residual shunt post PFO closure was assessed using transthoracic echocardiogram (TTE) with saline contrast. Cognitive function was evaluated using Montreal Cognitive Assessment (MoCA). tHcy levels in peripheral venous blood was measured using mass spectrometry.
Result
Among the patients, 67 (5.2%) developed VCID as measured by MoCA < 26 and NINDS AIREN criteria. The risk of VCID was significantly high in patients with residual shunt, particularly those with large shunt size (no shunt: 5.0%; small shunt: 6.4%; large shunt: 11.5%), suggesting a dose effect of PFO shunt (OR: 1.53; 95% CI: 1.09 ~ 2.16; p = 0.014) (Figure 1A, Table 1). Moreover, residual PFO shunt also contributed to tHcy elevation in circulation (β: 0.44; 95% CI: 0.19 ~ 0.69; p < 0.001) (Figure 1B, Table 1), and high tHcy levels were linked to increased risk of VCID (OR: 1.87; 95% CI: 1.14 ~ 3.07; p = 0.013) (Figure 1C, Table 1). Further mediation analyses revealed a significant indirect effect of PFO shunt on VCID through tHcy (OR: 1.29; 95% CI: 1.01 ~ 1.66; p = 0.047) (Figure 1D, Table 1). tHcy mediated 40% (95% CI: 6% ~ 74%; p = 0.021) of the total association between PFO shunt and VCID risk (Figure 1D, Table 1). These findings remained robust after adjusting for other VCID risk factors, such as age, diabetes, hyperlipidemia and hypertension (Table 1).
Conclusion
Our study suggested that that PFO shunt may contribute to the development of VCID by promoting circulatory tHcy elevation. Further mechanistic investigation of neurovascular injury and cognitive decline associated with PFO shunt is ongoing.
More abstracts on this topic:
Heart of the Mystery: Unveiling PFO's Role in Cryptogenic Stroke During Pregnancy
Bagha Zohaib, Ali Zohair, Wilson Sean, Grayver Evelina
Brain iron metabolism impairment differs between aged mouse models of cerebral hypoperfusion and iron overloadAshraf Areej, Monga Sheelu, Moruno-manchon Jose
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)