Final ID: 107
Polygenic risk prediction improves clinical prediction of atrial fibrillation diagnosed after stroke.
Abstract Body: Introduction:
Atrial fibrillation diagnosed after stroke (AFDAS) is common and differs from known atrial fibrillation (KAF), the most common cause of cardioembolic stroke. Because identification of AFDAS warrants administration of oral anticoagulants for secondary prevention, extended cardiac monitoring is often used, yet it is unclear who is most at risk for developing AFDAS. We aimed to determine whether prediction of AFDAS improves when adding genetic risk to clinical risk predictors in a real world clinical population of stroke survivors.
Methods
We retrospectively included patients with stroke between June 2003 and April 2017. Characteristics and outcomes were obtained from a data repository at Mass General Brigham (MGB), with chart review of AF diagnosis. Genome-wide polygenic risk scores (PRS) for AF were calculated using recent GWAS summary statistics. Clinical risk was determined using ReCHARGE-AF, a clinical post-stroke AF prediction score recalibrated from the CHARGE-AF score. For a subgroup, we obtained information on rare cardiomyopathy loss-of function genetic variants associated with AF risk. We determined whether patients had KAF, AFDAS or no AF, and used descriptive statistics to compare groups. We used Cox proportional hazards models to predict AFDAS, and calculated c-statistics to determine predictive validity at 1 month, 1 year and 5 years after stroke. We compared performance of clinical and genetic risk models through the DeLong test. We used 1,000-iteration bootstrapping to determine whether the addition of PRS improves model prediction.
Results
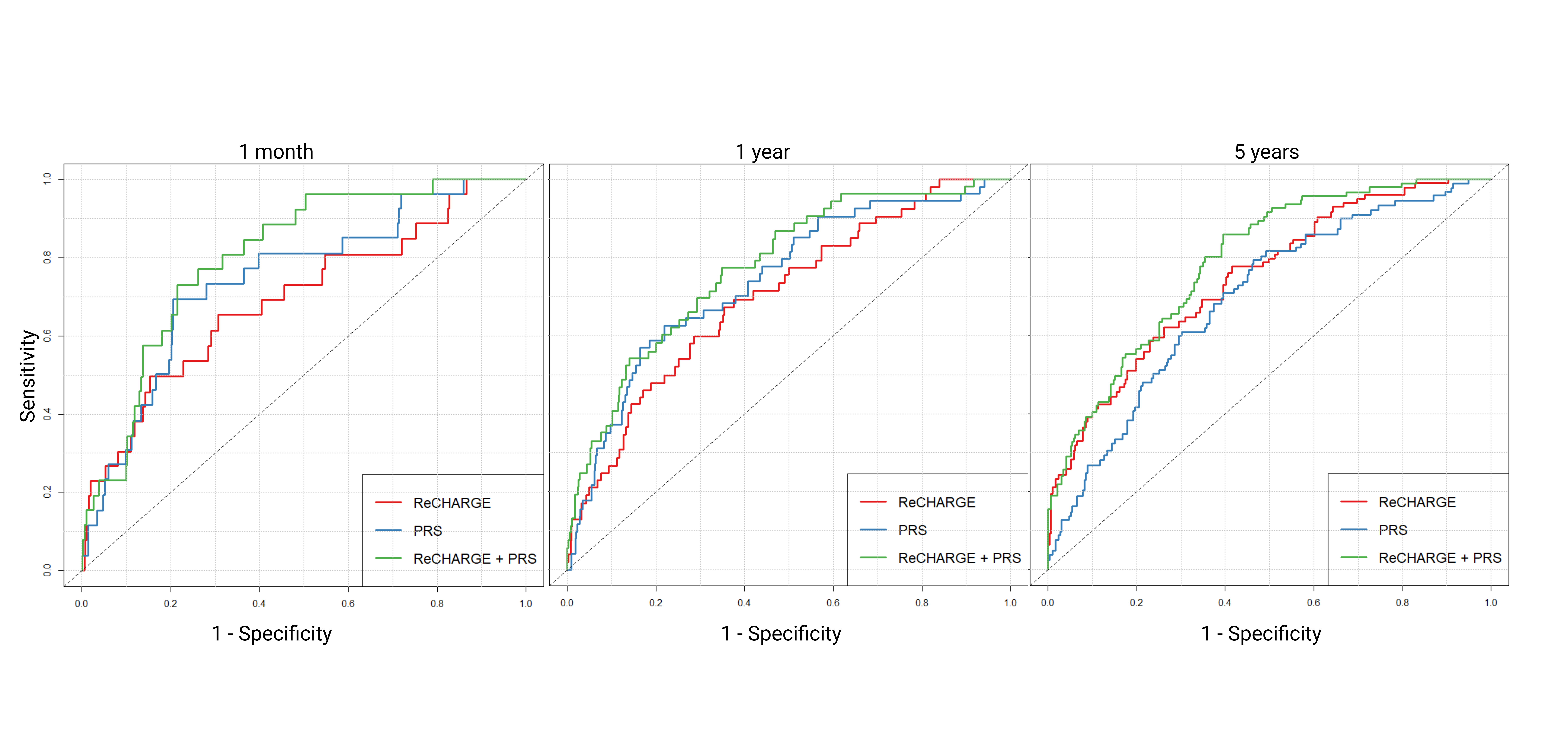
Of 1004 stroke patients, 900 (90%) were non-Hispanic white, with a mean age of 67. KAF was present in 239 (23.8%) patients, and 87 (8.7%) developed AFDAS. Patients with KAF and AFDAS had higher ReCHARGE-AF scores, higher rates of surface monitors and ILRs, and were more likely to have a high PRS, but did not differ in the presence of rare variants. Addition of PRS to clinical risk was associated with an increased performance at 30 days (AUC 0.784 vs 0.665, p < 0.01), 1 year (AUC 0.738 vs 0.659, p = 0.02) and 5 years (AUC 0.757 vs 0.708, p = 0.01). Addition of PRS was associated with improved discrimination, better reclassification, and a median improvement in risk score.
Conclusion
Prediction of AFDAS can be improved by adding polygenic risk scores to clinical risk prediction tools. Future studies are necessary to determine the feasibility of incorporating polygenic risk prediction for AFDAS.
Atrial fibrillation diagnosed after stroke (AFDAS) is common and differs from known atrial fibrillation (KAF), the most common cause of cardioembolic stroke. Because identification of AFDAS warrants administration of oral anticoagulants for secondary prevention, extended cardiac monitoring is often used, yet it is unclear who is most at risk for developing AFDAS. We aimed to determine whether prediction of AFDAS improves when adding genetic risk to clinical risk predictors in a real world clinical population of stroke survivors.
Methods
We retrospectively included patients with stroke between June 2003 and April 2017. Characteristics and outcomes were obtained from a data repository at Mass General Brigham (MGB), with chart review of AF diagnosis. Genome-wide polygenic risk scores (PRS) for AF were calculated using recent GWAS summary statistics. Clinical risk was determined using ReCHARGE-AF, a clinical post-stroke AF prediction score recalibrated from the CHARGE-AF score. For a subgroup, we obtained information on rare cardiomyopathy loss-of function genetic variants associated with AF risk. We determined whether patients had KAF, AFDAS or no AF, and used descriptive statistics to compare groups. We used Cox proportional hazards models to predict AFDAS, and calculated c-statistics to determine predictive validity at 1 month, 1 year and 5 years after stroke. We compared performance of clinical and genetic risk models through the DeLong test. We used 1,000-iteration bootstrapping to determine whether the addition of PRS improves model prediction.
Results
Of 1004 stroke patients, 900 (90%) were non-Hispanic white, with a mean age of 67. KAF was present in 239 (23.8%) patients, and 87 (8.7%) developed AFDAS. Patients with KAF and AFDAS had higher ReCHARGE-AF scores, higher rates of surface monitors and ILRs, and were more likely to have a high PRS, but did not differ in the presence of rare variants. Addition of PRS to clinical risk was associated with an increased performance at 30 days (AUC 0.784 vs 0.665, p < 0.01), 1 year (AUC 0.738 vs 0.659, p = 0.02) and 5 years (AUC 0.757 vs 0.708, p = 0.01). Addition of PRS was associated with improved discrimination, better reclassification, and a median improvement in risk score.
Conclusion
Prediction of AFDAS can be improved by adding polygenic risk scores to clinical risk prediction tools. Future studies are necessary to determine the feasibility of incorporating polygenic risk prediction for AFDAS.
More abstracts on this topic:
Coronary Artery Disease Polygenic Risk Scores with Equivalent Performance Produce Discordant Estimates of Individual Risk
Abramowitz Sarah, Voight Benjamin, Pasaniuc Bogdan, Levin Michael, Damrauer Scott, Boulier Kristin, Keat Karl, Cardone Katie, Shivakumar Manu, Depaolo John, Judy Renae, Kim Dokyoon, Ritchie Marylyn
A Rare Association of Darier’s Disease and Dilated Cardiomyopathy: A Case Report Exploring the Cardiac Implications of ATP2A2 MutationJacob Nidhi
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)