Final ID: WP347
Evaluating Brain Microvascular Endothelial Cell Dysfunction to Stenosis in a Tissue-Engineered Model of the Blood-Brain Barrier
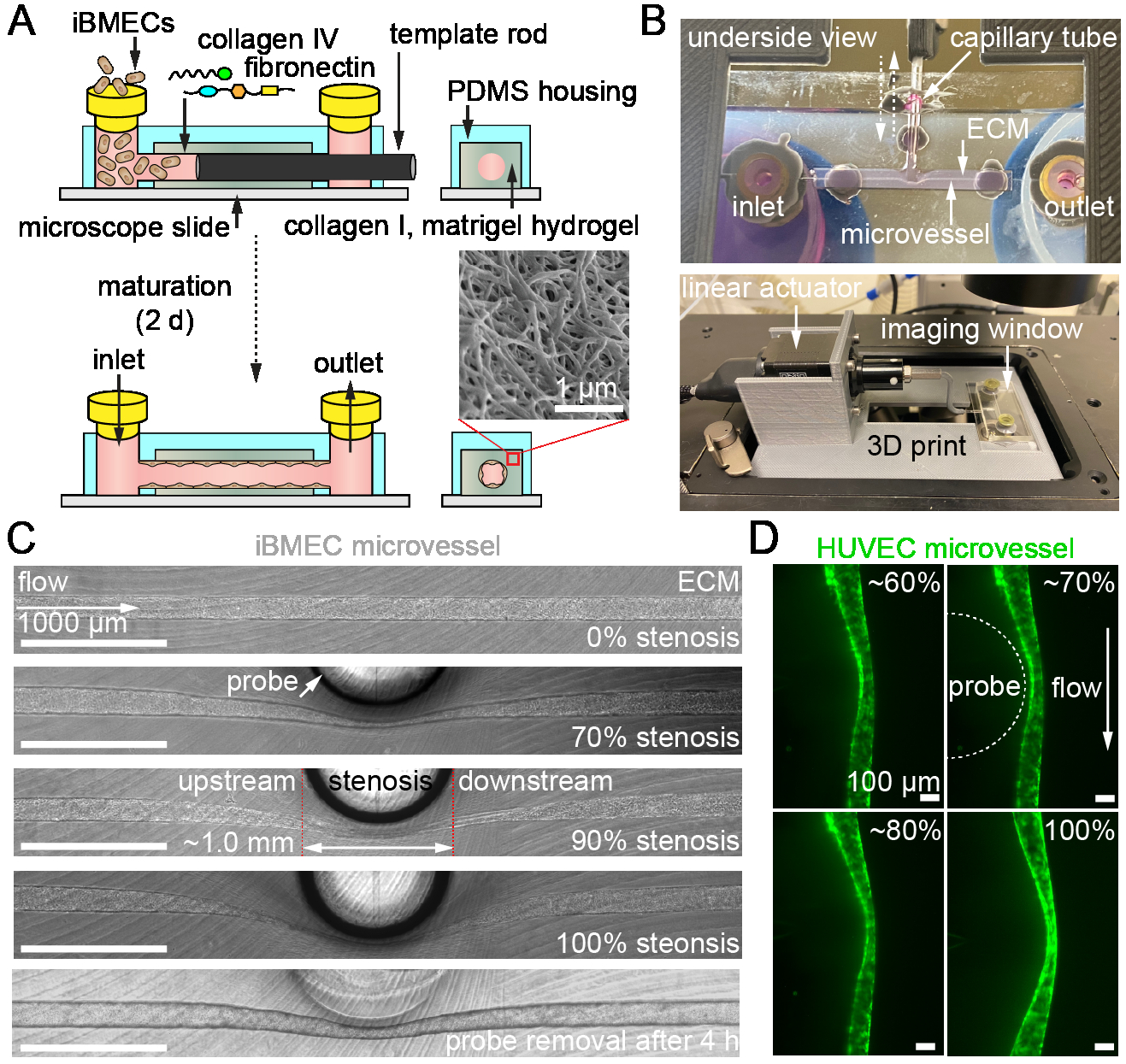
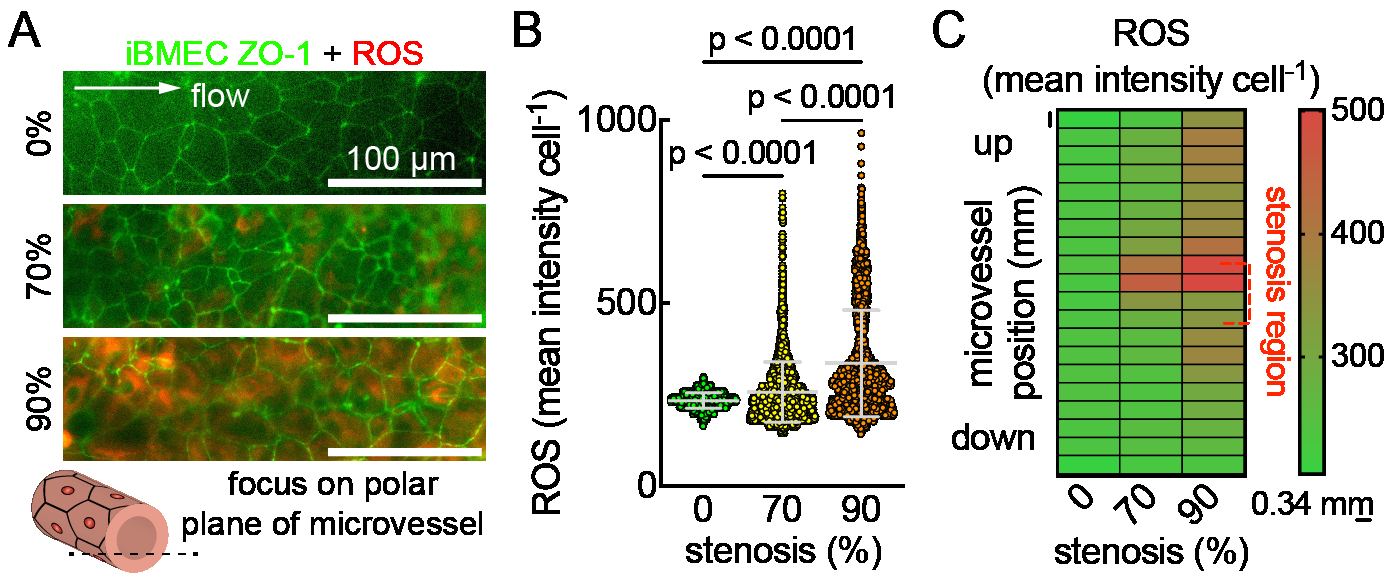
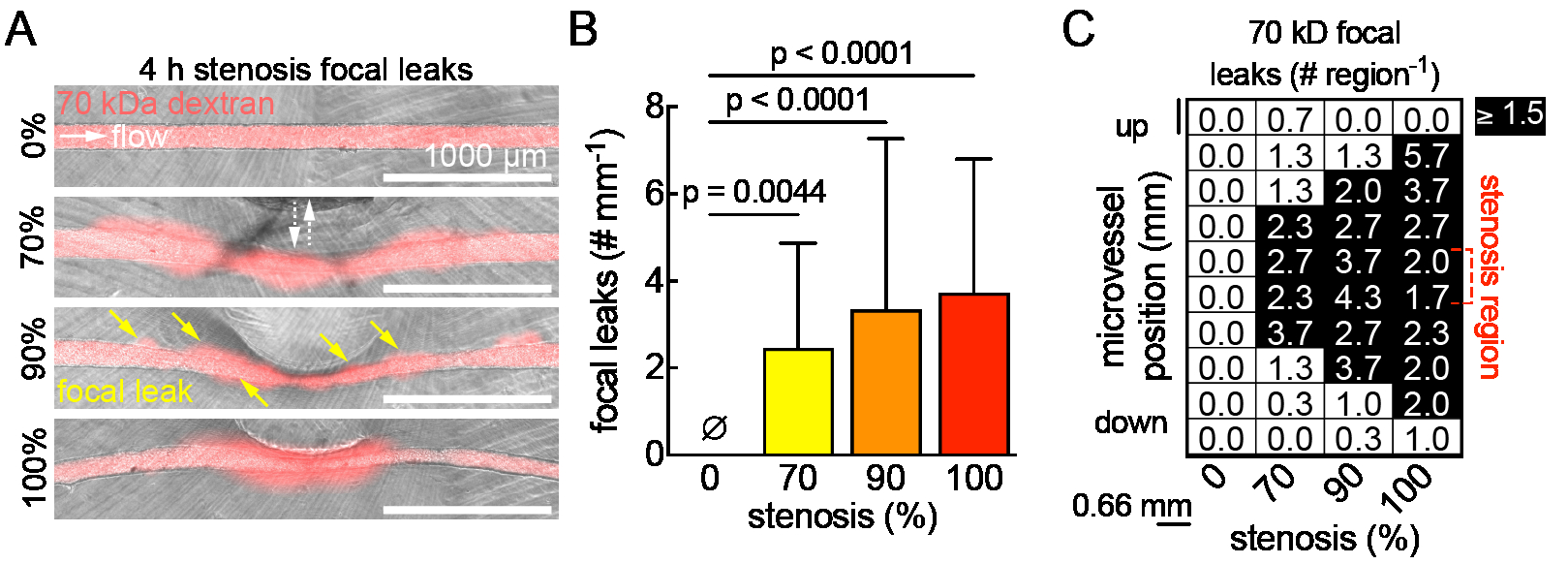
Methods: Pluripotent stem cells were differentiated into BMECs (iBMECs), validated for BBB phenotype, and seeded into 150 μm channels in collagen I/Matrigel hydrogels. Microvessels were matured for two days under physiological flow (~5 dyne cm-2). To model stenosis, a microactuator was mounted on a 3D printed platform and connected to a capillary tube (~1 mm diameter). Barrier function was probed by perfusing microvessels with Lucifer yellow + 70 kDa dextran after 4 h of varied stenosis %, followed by thresholding in ImageJ to track focal leaks. Reactive oxygen species (ROS) generation was assessed using a live cell ROS stain and imaging on the microvessel polar planes with an epifluorescent microscope. The zonula occludens-1 (ZO-1) fluorescent tag on the iBMECs outlined cells for single-cell analyses in CellProfiler using CellPose.
Results and Conclusions: A tissue-engineered BBB model from the Searson Lab was modified (Fig. 1A) to include a microactuator for inducing adjustable (0-100%) and reversible stenosis during the perfusion of iBMEC & HUVEC microvessels within a hydrogel ECM (Fig. 1B, C). Preliminary results show focal leaks forming immediately after stenosis (Fig. 2A, B), with distribution increasing along the microvessel length as stenosis intensifies (Fig. 2C). Using ZO-1 labeled iBMECs for cell segmentation from epifluorescence images, we found that ROS significantly increases with greater stenosis (Fig. 3A, B), particularly in the center and upstream of microvessels (Fig. 3C). In conclusion, our findings suggest that stenosis disrupts barrier function, with the disruption not always being regionally dependent. We will further investigate whether these changes are due to physical or chemical factors, such as altered wall shear stress or oxygen/glucose bottlenecks.
More abstracts on this topic:
Dittrich Gesine, Xu Xingbo, Riester Daniel, Meyer Tim, Tiburcy Malte, Fischer Andre, Zimmermann Wolfram
A Real-World Pilot for Diagnostic Yield of Cardiac CTA vs Echocardiography in Acute Ischemic StrokeChakravarthula Nitin Ramanujam, Milani Marcus, Tessmer Megan, Staugaitis Abbey, Akimoto Kai, Markowitz Jeremy, Kalra Rajat, Nijjar Prabhjot, Streib Christopher
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.