Final ID: TH127
Detecting Potential Threshold Bias in Recorded Blood Pressure in Clinical Practice and Research: A study of 7 International Datasets
Aim: To develop a method for detecting potential threshold bias using population-based surveillance data as a reference and apply it to research cohort, clinical practice, and clinical trial datasets.
Hypothesis: Threshold bias may be present in settings with a BP control goal (e.g., hypertension programs and some clinical trials), but not in those without (e.g., research cohorts).
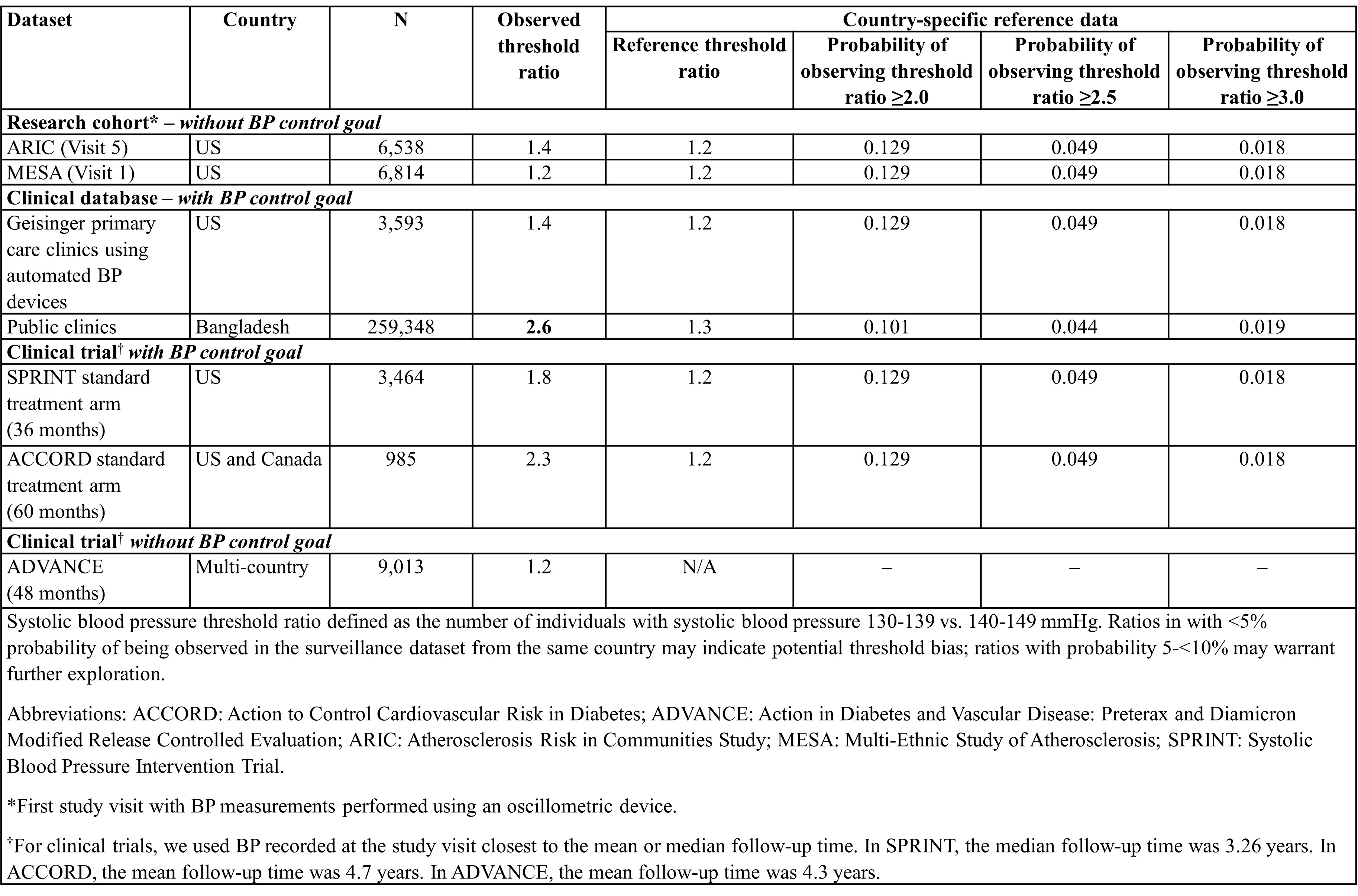
Methods: In national surveillance datasets (i.e., reference datasets) from the US and Bangladesh, we examined the systolic blood pressure (SBP) distributions in adults with treated hypertension and calculated the expected SBP threshold ratios, defined as the number of individuals with SBP 130-139 vs. 140-149 mmHg. We used weighted bootstrapping with 10,000 random draws of 100 observations to obtain the probability of observing prespecified threshold ratios (e.g., ≥2.0, ≥2.5, ≥3.0) in the reference datasets. We then calculated the SBP threshold ratios in 7 research cohort, clinical practice, and clinical trial datasets and compared them to the bootstrap probabilities from the reference datasets from the same country as a scale to indicate the likelihood of threshold bias.
Results: The SBP threshold ratios in the reference datasets for the US and Bangladesh were 1.2 and 1.3, respectively. In each reference dataset, the probability of observing ratios ≥2.5 was <5% and ≥3.0 was <2%; thus, ratios exceeding these values in other datasets may indicate potential threshold bias. When we calculated threshold ratios in 2 research cohorts, there was no evidence of threshold bias (Table). Among clinical datasets, we observed threshold ratios of 2.6 in public clinics in Bangladesh, which may indicate threshold bias. One clinical trial (ACCORD) with a BP goal had a threshold ratio of 2.3; another trial without a BP goal (ADVANCE) had a threshold ratio of 1.2.
Conclusion: Using our proposed method, we identified multiple clinical practice and trial datasets with BP control goals with potential threshold bias. The results highlight the need for routine monitoring of threshold bias and renewed emphasis on obtaining high-quality BP measurements, including accurate recording.
- Foti, Kathryn ( University of Alabama at Birmingham , Birmingham , Alabama , United States )
- Chang, Alex ( Geisinger Clinic , Danville , Pennsylvania , United States )
- Harris, Katie ( The George Institute for International Health , Sydney , New South Wales , Australia )
- Woodward, Mark ( The George Institute for International Health , Sydney , New South Wales , Australia )
- Amin, Mohammad Robed ( Directorate of General Health Services , Dhaka , Bangladesh )
- Hossain, Syed ( Directorate of General Health Services , Dhaka , Bangladesh )
- Bhuiyan, Mahfuzur Rahman ( National Heart Foundation , Dhaka , Bangladesh )
- Jubayer, Shamim ( National Heart Foundation , Dhaka , Bangladesh )
- Choudhury, Sohel ( National Heart Foundation Hospital , Dhaka , Bangladesh )
- Girma, Dessie ( Resolve to save lives , Addis Ababa , Ethiopia )
- Dereje, Betsegaw ( Resolve to save lives , Addis Ababa , Ethiopia )
- Marklund, Matti ( Johns Hopkins University , Baltimore , Maryland , United States )
- Gupta, Reena ( University of California San Francisco , San Francisco , California , United States )
- Appel, Lawrence ( JOHNS HOPKINS UNIVERSITY , Ellicott City , Maryland , United States )
- Matsushita, Kunihiro ( Johns Hopkins Bloomberg School of Public Health , Baltimore , Maryland , United States )
- Zhao, Di ( Johns Hopkins University , Baltimore , Maryland , United States )
- Pathiravasan, Chathurangi ( Johns Hopkins University , Baltimore , Maryland , United States )
- Shen, Ziling ( Johns Hopkins University , Baltimore , Maryland , United States )
- Blasco-colmenares, Elena ( JOHNS HOPKINS UNIVERSITY , Baltimore , Maryland , United States )
- Miller, Edgar ( JOHNS HOPKINS MEDICAL INSTITUTIONS , Baltimore , Maryland , United States )
- Nordberg, Cara ( Geisinger , Danville , Pennsylvania , United States )
- Hirsch, Annemarie ( Geisinger , Danville , Pennsylvania , United States )
Meeting Info:
Session Info:
Poster Session 1 and Reception (includes TAC Poster Competition)
Thursday, 09/04/2025 , 05:30PM - 07:00PM
Poster Session
More abstracts on this topic:
Hussain Abdur-rehman, Soni Ashesh, Stead Thor, Persaud Nadiya, Ganti Latha
A Study Using Team Based Hypertension Care Focused on Black Patients to Inform a Future Randomized TrialManandhar Srista, Taylor Yhenneko, Bosworth Hayden, Pokharel Yashashwi, Chhetri Sunit, Sutton Danielle, Saha Animita, Kaur Suneet, Brown Josh, Moore Justin, Callahan Kate, Williamson Jeff
More abstracts from these authors:
Ogungbe Bunmi, Commodore-mensah Yvonne, Brady Tammy, Appel Lawrence, Matsushita Kuni, Marklund Matti, Hu Xiao, Hussain Tasfia, Nguyen Binh, Abrar Ahmad, Jubayer Shamim, Choudhury Sohel, Bhuiyan Mahfuzur Rahman, Moran Andrew
The Use of Automated Blood Pressure Measurement Devices is Associated with Decreased Threshold Bias for Systolic Blood Pressure in a Large Healthcare System in Central and Northeastern PennsylvaniaNordberg Cara, Foti Kathryn, Zhao Di, Matsushita Kunihiro, Hirsch Annemarie, Chang Alexander