Final ID: P-302
Impact of Publication of Hypertension Guidelines on Inappropriate Use of Intravenous (IV) Antihypertensive Medications for Hypertensive Urgencies (HU)
Abstract Body: Background: Hypertensive urgencies are common in hospitalized patients. In late 2017 and 2018, the publication of major U.S and European hypertension guidelines included specific recommendations against the use of IV antihypertensive medications for the management of severe acute BP elevations in the absence of acute target organ damage (HU).
Aim: To study the impact of the publication of guidelines on local utilization of IV medications for HU.
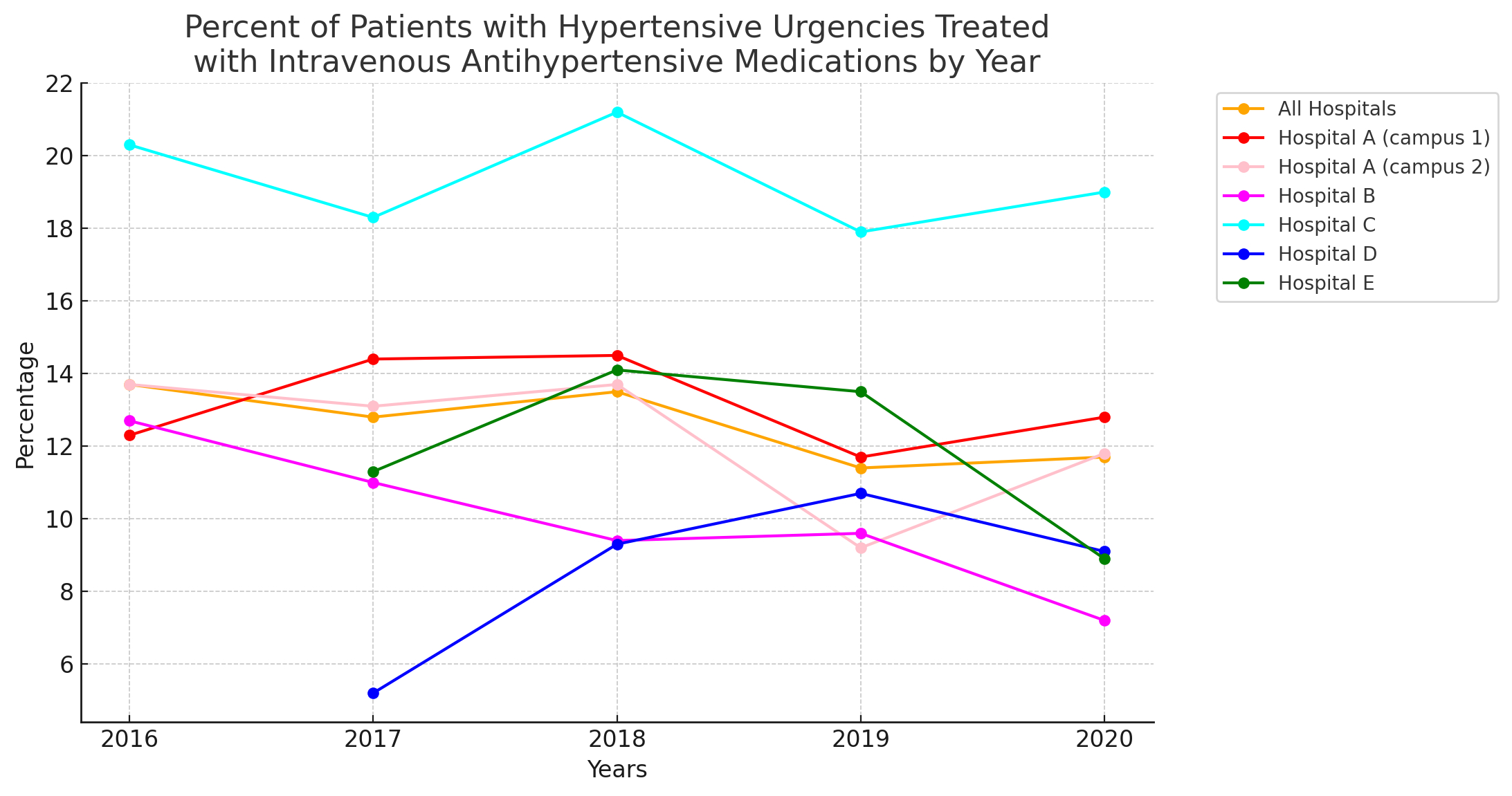
Methods and Results: We analyzed 224,265 non-ICU, non-obstetric, non-pediatric hospital admissions to 5 U.S. hospitals (total 2,681 beds) in a medium-sized U.S. healthcare system between January 2016 and March 2020. These included one large academic medical center with two campuses (Hosp A), one medium sized teaching community hospital (Hosp B), one small teaching community hospital (Hosp C), and two small non-teaching hospitals (Hosp D and E). Among all admitted patients, 22,929 (10.2%) developed HU (SBP>180 or DBP >110 mmHg sustained for at least 1 hour without evidence of acute target organ damage). A total of 2,914 HU patients (12.7%) received at least 1 intravenous medication within 6 hours of acute BP elevation, most commonly hydralazine (N 1473, 50.5%], labetalol (N 749, 25.7%) or metoprolol (N 632, 21.7%). The fraction of patients receiving IV antihypertensives decreased during the five years of the study across the five hospitals, but the absolute change was small (2016=13.7%, 2017=12.8%, 2018=12.5%, 2019=11.4%, 2020=11.7%, P value for overall change = 0.003). The Figure displays temporal trends for each hospital. There was significant heterogeneity across hospitals (P<0.0001). Trends were not related to teaching (vs. nonteaching) status of each hospital.
Conclusion: In a medium sized U.S. hospital system, the publication of guidelines recommending against IV antihypertensive use in HU was temporally associated with very modest reductions in IV medication use in HU. Process improvement initiatives are needed to optimize guideline-congruent practice.
Aim: To study the impact of the publication of guidelines on local utilization of IV medications for HU.
Methods and Results: We analyzed 224,265 non-ICU, non-obstetric, non-pediatric hospital admissions to 5 U.S. hospitals (total 2,681 beds) in a medium-sized U.S. healthcare system between January 2016 and March 2020. These included one large academic medical center with two campuses (Hosp A), one medium sized teaching community hospital (Hosp B), one small teaching community hospital (Hosp C), and two small non-teaching hospitals (Hosp D and E). Among all admitted patients, 22,929 (10.2%) developed HU (SBP>180 or DBP >110 mmHg sustained for at least 1 hour without evidence of acute target organ damage). A total of 2,914 HU patients (12.7%) received at least 1 intravenous medication within 6 hours of acute BP elevation, most commonly hydralazine (N 1473, 50.5%], labetalol (N 749, 25.7%) or metoprolol (N 632, 21.7%). The fraction of patients receiving IV antihypertensives decreased during the five years of the study across the five hospitals, but the absolute change was small (2016=13.7%, 2017=12.8%, 2018=12.5%, 2019=11.4%, 2020=11.7%, P value for overall change = 0.003). The Figure displays temporal trends for each hospital. There was significant heterogeneity across hospitals (P<0.0001). Trends were not related to teaching (vs. nonteaching) status of each hospital.
Conclusion: In a medium sized U.S. hospital system, the publication of guidelines recommending against IV antihypertensive use in HU was temporally associated with very modest reductions in IV medication use in HU. Process improvement initiatives are needed to optimize guideline-congruent practice.
More abstracts on this topic:
Altering Cardiovascular Mortality in HFpEF with SGLT2i or ARNI — A Head-to-Head Analysis
Hariyanto Jesslyn, Veera Chirag, Lenzi Pinto Manoela, Chatterjee Anoushka, Eltawansy Sherif
A cerebrovascular longitudinal atlas: different rates of morphological change in aneurysm patients associated with hypertension and diabetesChien Aichi, Salamon Noriko, Vinuela Fernando, Szeder Viktor, Colby Geoffrey, Jahan Reza, Boyle Noel, Villablanca Juan, Duckwiler Gary