Final ID: P2013
Underrepresentation of African Countries in Randomized Controlled Trials: A 2022 Analysis Across Five Leading General Medical Journals
Abstract Body: Abstract
Objective: To evaluate the representation of African countries in randomized controlled trials (RCTs) published in five leading general medical journals in 2022.
Methods: This systematic review assessed RCTs involving human subjects published between January 1, 2022, and December 31, 2022, across The New England Journal of Medicine, The Lancet, JAMA, Nature Medicine, and BMJ. Trials included were conducted in diverse settings, encompassing hospitals, research institutions, and community health centers. Geographic locations and participant demographics were extracted for analysis. The primary outcome was the proportion of RCTs conducted solely within African countries. The secondary outcome assessed African representation in multi-continental RCTs. Data were aggregated to determine the percentage of Africa-exclusive RCTs and African representation in multi-continental trials within each journal.
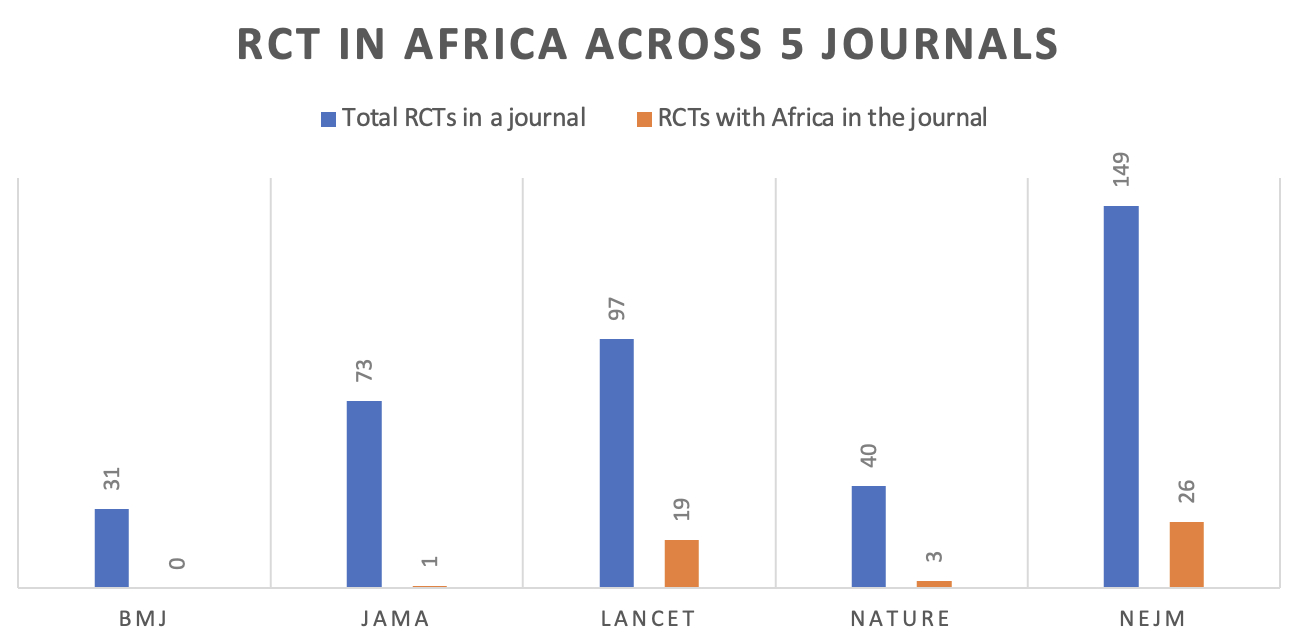
Results: Of the 390 RCTs meeting inclusion criteria (BMJ: 31, JAMA: 73, The Lancet: 97, Nature Medicine: 40, NEJM: 149), only 1.6% were conducted exclusively in Africa, with an additional 6.9% representing multi-continental studies where at least one African location was included. A total of 9 Africa-exclusive RCTs were published in 2022: The Lancet (4.1%), NEJM (2.7%), and Nature Medicine (2.5%). No Africa-exclusive RCTs appeared in BMJ or JAMA. Of multi-continental RCTs, NEJM included 14.8% with African sites, followed by The Lancet (9.3%), Nature Medicine (5.0%), and JAMA (1.4%). The BMJ published no RCTs with African representation. Within Africa, study sites were unevenly distributed. South Africa accounted for 67.5% of African RCTs, Northern Africa (7 countries) contributed 8.2%, Eastern Africa (22 countries) contributed 51.0%, Western Africa (17 countries) contributed 22.4%, Southern Africa (5 countries) contributed 75.5%, and Central Africa (9 countries) accounted for only 2.0% of the African representation.
Conclusion: This analysis reveals a significant underrepresentation of African countries in RCTs published in top medical journals in 2022. The findings underscore the critical need for increased African participation in RCTs. Expanding African representation is essential for the generalizability of clinical research and the promotion of equitable health outcomes globally.
Objective: To evaluate the representation of African countries in randomized controlled trials (RCTs) published in five leading general medical journals in 2022.
Methods: This systematic review assessed RCTs involving human subjects published between January 1, 2022, and December 31, 2022, across The New England Journal of Medicine, The Lancet, JAMA, Nature Medicine, and BMJ. Trials included were conducted in diverse settings, encompassing hospitals, research institutions, and community health centers. Geographic locations and participant demographics were extracted for analysis. The primary outcome was the proportion of RCTs conducted solely within African countries. The secondary outcome assessed African representation in multi-continental RCTs. Data were aggregated to determine the percentage of Africa-exclusive RCTs and African representation in multi-continental trials within each journal.
Results: Of the 390 RCTs meeting inclusion criteria (BMJ: 31, JAMA: 73, The Lancet: 97, Nature Medicine: 40, NEJM: 149), only 1.6% were conducted exclusively in Africa, with an additional 6.9% representing multi-continental studies where at least one African location was included. A total of 9 Africa-exclusive RCTs were published in 2022: The Lancet (4.1%), NEJM (2.7%), and Nature Medicine (2.5%). No Africa-exclusive RCTs appeared in BMJ or JAMA. Of multi-continental RCTs, NEJM included 14.8% with African sites, followed by The Lancet (9.3%), Nature Medicine (5.0%), and JAMA (1.4%). The BMJ published no RCTs with African representation. Within Africa, study sites were unevenly distributed. South Africa accounted for 67.5% of African RCTs, Northern Africa (7 countries) contributed 8.2%, Eastern Africa (22 countries) contributed 51.0%, Western Africa (17 countries) contributed 22.4%, Southern Africa (5 countries) contributed 75.5%, and Central Africa (9 countries) accounted for only 2.0% of the African representation.
Conclusion: This analysis reveals a significant underrepresentation of African countries in RCTs published in top medical journals in 2022. The findings underscore the critical need for increased African participation in RCTs. Expanding African representation is essential for the generalizability of clinical research and the promotion of equitable health outcomes globally.
More abstracts on this topic:
A Polypill Strategy for Lipid Lowering and Anti-Platelet Therapy After Acute Coronary Syndrome: A Pilot Randomized Controlled Trial
Keshvani Neil, Wang Thomas, Pandey Ambarish, Coellar Juan David, Rizvi Syed Kazim, Jain Anand, Bustillo-rubio M. Karina, Segar Matthew, Lokesh Nidhish, Miller James, Yates Sean
A First-In-Human Phase 1 Study of the Safety, Tolerability, and Pharmacodynamics of REGN7544, a Novel Natriuretic Peptide Receptor 1–Blocking Monoclonal AntibodyAhmed Mohsin, Morton Lori, Olenchock Benjamin, Herman Gary, Wynne Chris, Marin Ethan, Tuckwell Katie, Xu Meng, Cheng Xiping, Redington Emily, Koyani Bharatkumar, Mateo Katrina, Thakur Mazhar, Devalaraja-narashimha Kishor