Final ID: Tu069
Development of a Novel Biallelic, Haploinsufficient Mouse Model of MYBPC3 Related Hypertrophic Cardiomyopathy
Abstract Body: Background: No current animal models re-capitulate MyBP-c haploinsufficiency and hypertrophic cardiomyopathy (HCM) observed in patients with heterozygous MYBPC3 truncating variants, the most common genetic cause of HCM. We recently identified a hypomorphic (partial) loss of function MYBPC3 variant that increases disease severity in HCM patients when combined with an MYBPC3 truncating variant.
Hypothesis: Additive effects from a hypomorphic loss of function MYBPC3 variant observed in human HCM will result in MyBP-c protein haploinsufficiency and HCM in a biallelic murine model.
Methods: To test MYBPC3 dose effects, induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) were first derived from an HCM patient with biallelic hypomorphic and complete loss of function variants in MYBPC3. We then generated Mybpc3 knock out (Mybpc3KO) and hypomorphic knock in (Mybpc3KI) alleles in mice. Control (Mybpc3+/+), monoallelic, and biallelic mice and iPSC-CMs were characterized with morphometric measurements, echocardiography, mass spectroscopy, and RNA sequencing.
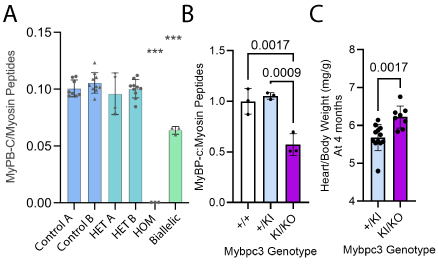
Results: Biallelic iPSC-CMs demonstrated haploinsufficiency not present in heterozygous models through dose reduction in MYBPC3 protein (Fig 1A). Similarly, biallelic Mybpc3KI/KO mice had a reduction in Mybpc3 transcripts (62±3%, p<0.001) with 43±11%% reduction in MyBP-c protein (Fig 1B) and cardiac hypertrophy (10±5% increase in heart mass, p=0.002, Fig 1C).
Conclusions: Graded reduction in Mybpc3 transcripts in a biallelic mouse model results in MyBP-c haploinsufficiency similar to that observed in HCM patients. Corresponding hypertrophy establishes this approach as a clinically relevant, novel model of MYBPC3 HCM.
Hypothesis: Additive effects from a hypomorphic loss of function MYBPC3 variant observed in human HCM will result in MyBP-c protein haploinsufficiency and HCM in a biallelic murine model.
Methods: To test MYBPC3 dose effects, induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) were first derived from an HCM patient with biallelic hypomorphic and complete loss of function variants in MYBPC3. We then generated Mybpc3 knock out (Mybpc3KO) and hypomorphic knock in (Mybpc3KI) alleles in mice. Control (Mybpc3+/+), monoallelic, and biallelic mice and iPSC-CMs were characterized with morphometric measurements, echocardiography, mass spectroscopy, and RNA sequencing.
Results: Biallelic iPSC-CMs demonstrated haploinsufficiency not present in heterozygous models through dose reduction in MYBPC3 protein (Fig 1A). Similarly, biallelic Mybpc3KI/KO mice had a reduction in Mybpc3 transcripts (62±3%, p<0.001) with 43±11%% reduction in MyBP-c protein (Fig 1B) and cardiac hypertrophy (10±5% increase in heart mass, p=0.002, Fig 1C).
Conclusions: Graded reduction in Mybpc3 transcripts in a biallelic mouse model results in MyBP-c haploinsufficiency similar to that observed in HCM patients. Corresponding hypertrophy establishes this approach as a clinically relevant, novel model of MYBPC3 HCM.
More abstracts on this topic:
Cross-Platform Proteomics and Machine Learning Algorithms Nominate Biomarkers of Atrial Fibrillation in Stroke Patients: An Exploratory Study
Misra Shubham, Natu Aditya, Kumar Prateek, Watson Caroline, Frankel Michael, Rangaraju Srikant
A Suppression-and-Replacement Platform Identifies Pathogenic Variants in the LMNA-Encoded Lamin A/C Ig-Like Domain as Drivers of Nuclear Distortion and AggregationHuynh Trung, Kim Changsung, Tester David, Castrichini Matteo, Giudicessi John, Ackerman Michael