Final ID:
The Need for Ongoing Oral Anticoagulation in Patients with Clinical Stroke Risk Factors After Successful Catheter Ablation of Atrial Fibrillation: The OCEAN Randomized Trial
BACKGROUND
It is unknown whether successful catheter ablation of atrial fibrillation (AF) reduces AF burden sufficiently to reduce stroke risk and obviate the need for long-term oral anticoagulation (OAC). Current guidelines recommend indefinite OAC treatment after AF ablation guided by stroke risk and not on the apparent success of the procedure. However, this recommendation is not based on any randomized data. The OCEAN trial hypothesized that a strategy of continued OAC will be superior to antiplatelet therapy for reducing the risk of stroke, systemic embolism, or covert embolic stroke after successful ablation of AF.
METHODS
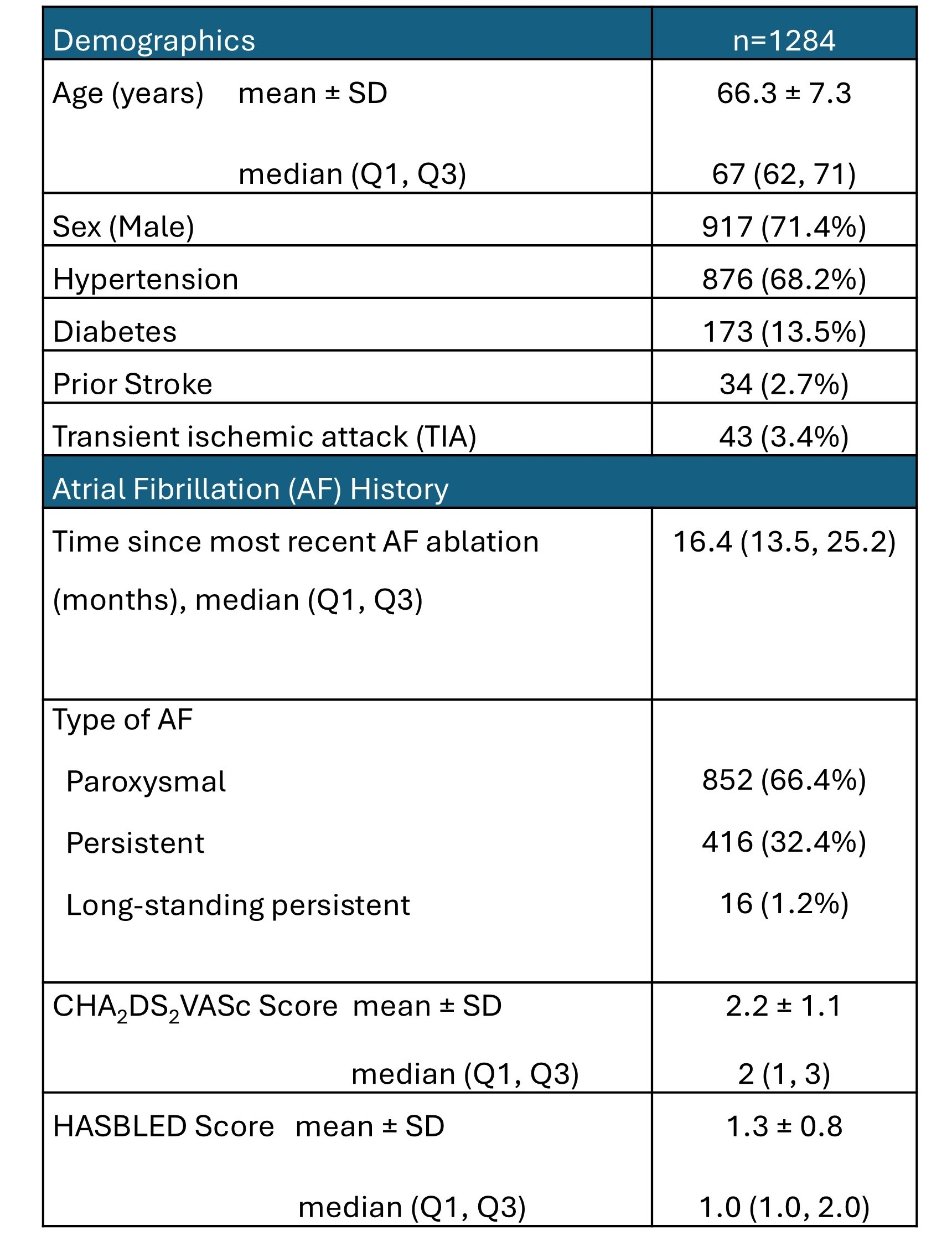
The OCEAN trial (NCT02168829) is a multicenter, international, prospective, randomized, open-label, blinded endpoint trial (1:1 randomization). Inclusion criteria were patients (1) greater than one-year post-successful catheter ablation(s) without evidence of any clinically apparent arrhythmia recurrence and (2) CHA2DS2-VASc risk score ≥1 or ≥2 (if female sex or vascular disease were a risk factor). Patients were randomized to either antiplatelet therapy (acetylsalicylic acid 75-120 mg daily) or oral anticoagulation (rivaroxaban 15 mg daily). Patients were followed up for 3 years. All patients had protocol-mandated cerebral magnetic resonance imaging (MRI) after enrollment and 3 years after randomization. The primary efficacy outcome was a composite of stroke, systemic embolism, and covert embolic stroke. Covert embolic stroke was defined as one or more new infarcts ≥15 mm detected between the baseline and 3-year cerebral MRI.
RESULTS
We randomized 1284 patients between March 30, 2016 and July 25, 2022 at 56 centers (5 Australia, 16 Belgium, 22 Canada, 1 China, 11 Germany & 1 Israel). Baseline demographics are shown in the table. Enrollment was stopped early by the data safety and monitoring board. Patients were followed for a median of 36 months. Anticipated database lock is August 15, 2025, 36 months after enrollment of the last patient. Results of the primary efficacy endpoint and safety endpoint (bleeding) will be presented.
CONCLUSION
OCEAN will be the first randomized trial to evaluate whether ongoing OAC is necessary after successful AF ablation in patients with stroke risk factors.
- Verma, Atul ( McGill University Health Centre , Montreal , Quebec , Canada )
- Essebag, Vidal ( McGill University Health Centre , Montreal , Quebec , Canada )
- Champagne, Jean ( Institut Universitaire de Cardiologie et de Pneumologie de Quebec , Quebec City , Quebec , Canada )
- Hill, Michael ( UNIVERSITY CALGARY , Calgary , Alberta , Canada )
- Smith, Eric ( UNIVERSITY OF CALGARY , Calgary , Alberta , Canada )
- Wells, George ( University of Ottawa, Heart Inst. , Ottawa , Ontario , Canada )
- Birnie, David ( UNIV OF OTTAWA HEART INSTITUTE , Ottawa , Ontario , Canada )
- Jiang, Chenyang ( Sir Run Run Shaw Hospital , Hangzhou , China )
- Heidbuchel, Hein ( University of Antwerp , Antwerp , Belgium )
- Hindricks, Gerhard ( Charité – Universitätsmedizin Berlin , Berlin , Germany )
- Kirchhof, Paulus ( UKE Hamburg , Hamburg , Germany )
- Healey, Jeff ( McMaster University , Hamilton , Ontario , Canada )
- Sharma, Mike ( McMaster University , Hamilton , Ontario , Canada )
- Ha, Andrew ( Peter Munk Cardiac Center , Toronto , Ontario , Canada )
Meeting Info:
Session Info:
Dilemmas in Antithrombotic Therapy in AF Care Post Procedures
Saturday, 11/08/2025 , 01:30PM - 02:45PM
Late-Breaking Science
More abstracts on this topic:
Beavis James, Goncer Timothy
A ChatGLM-based stroke diagnosis and prediction toolSong Xiaowei, Wang Jiayi, Ma Weizhi, Wu Jian, Wang Yueming, Gao Ceshu, Wei Chenming, Pi Jingtao
More abstracts from these authors:
Healey Jeff
Sympathetic Denervation as a Substrate for Persistent Atrial Fibrillation Assessed Using 11C-Hydroxyephedrine PET ImagingDai Yuchen, Wells George, Beanlands Rob, Birnie David, Dekemp Robert, Nery Pablo, Tavoosi Anahita, Nair Girish, Redpath Calum, Golian Mehrdad, Thornhill Rebecca, Pena-fernandez Elena, Hansom Simon, Sadek Mouhannad