Final ID: 4392958
Implantable Epicardial Cell-Based Platform for Localized Immunomodulation of Ischemic Myocardium
Abstract Body (Do not enter title and authors here): Background:
Ischemic myocardium increases inflammatory macrophages which is believed to exacerbate deleterious ventricular remodeling and worsen ventricular function. While current therapeutics focus on platelet inhibition, reducing hemodynamic stress, and revascularization, few interventions target immunomodulation. Cytokine-based therapies are promising options such as Interleukin-10 (IL10) which can regulate local immune response. However, systemic delivery of cytokines is challenging due to off-target toxicity, short half-lives, and poor distribution to diseased tissues. To overcome these limitations, we engineered a 3D printed cell-based platform for local myocardial cytokine therapy in a rat MI model.
Research Question:
This study asks if local cytokine delivery can promote immunomodulation after myocardial infarction.
Methods:
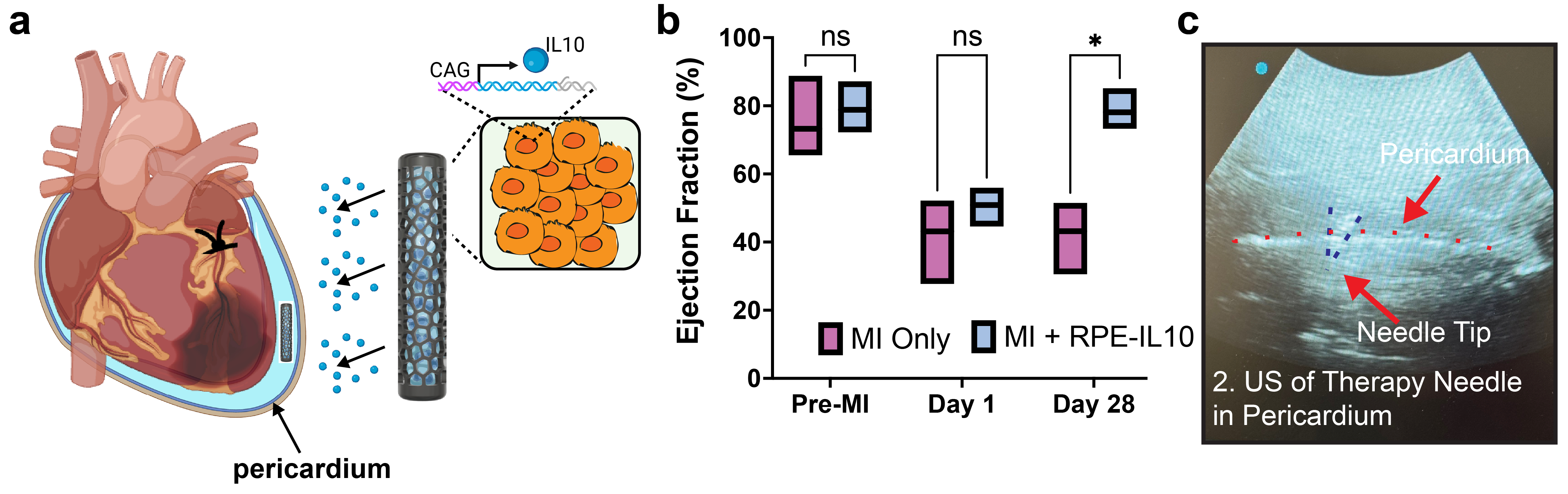
We 3D printed a lattice scaffold that housed engineered retinal pigment epithelium cells expressing IL10 (termed VitaGrafts) embedded in alginate hydrogel. VitaGrafts were implanted via left thoracotomy and MI was induced by LAD ligation (N=6). Pre- and post-implant VitaGrafts production was assessed by ELISA. Heart function was assessed with echocardiography and histology. The cellular landscape was assessed by cytometry time of flight (CyTOF) and single cell RNA sequencing (scRNAseq). Lastly, to assess clinical translation feasibility, VitaGrafts were instilled into the pericardial space with a catheter in a pig cadaver.
Results:
VitaGrafts delivered IL10 to heart tissue over a dynamic range. Echocardiography of rats with treatment for 28 days vs rats with MI showed ~2-fold improvement in ejection fraction (EF). EF improvement was further validated by a ~4-fold reduction in fibrosis (~18% vs ~5%, p<0.0001) seen on histology. IL10 VitaGraft increased reparative M2 macrophages 108-fold (p<0.001) and reduced Cytotoxic T cells 36% on CyTOF. This was confirmed with scRNAseq and demonstrated downregulation of inflammatory genes including the IFN-g pathway. Lastly, we demonstrated the VitaGraft could be delivered percutaneously in a pig cadaver using a pericardial catheter.
Conclusions:
Local delivery of IL10 to ischemic myocardium increases anti-inflammatory macrophages and increases reparative phenotypes. This results in improved ventricular function and reduced fibrosis after MI. The VitaGraft offers a bioengineering cell-based platform to deliver local cytokine therapy that can be inserted minimally invasively.
Ischemic myocardium increases inflammatory macrophages which is believed to exacerbate deleterious ventricular remodeling and worsen ventricular function. While current therapeutics focus on platelet inhibition, reducing hemodynamic stress, and revascularization, few interventions target immunomodulation. Cytokine-based therapies are promising options such as Interleukin-10 (IL10) which can regulate local immune response. However, systemic delivery of cytokines is challenging due to off-target toxicity, short half-lives, and poor distribution to diseased tissues. To overcome these limitations, we engineered a 3D printed cell-based platform for local myocardial cytokine therapy in a rat MI model.
Research Question:
This study asks if local cytokine delivery can promote immunomodulation after myocardial infarction.
Methods:
We 3D printed a lattice scaffold that housed engineered retinal pigment epithelium cells expressing IL10 (termed VitaGrafts) embedded in alginate hydrogel. VitaGrafts were implanted via left thoracotomy and MI was induced by LAD ligation (N=6). Pre- and post-implant VitaGrafts production was assessed by ELISA. Heart function was assessed with echocardiography and histology. The cellular landscape was assessed by cytometry time of flight (CyTOF) and single cell RNA sequencing (scRNAseq). Lastly, to assess clinical translation feasibility, VitaGrafts were instilled into the pericardial space with a catheter in a pig cadaver.
Results:
VitaGrafts delivered IL10 to heart tissue over a dynamic range. Echocardiography of rats with treatment for 28 days vs rats with MI showed ~2-fold improvement in ejection fraction (EF). EF improvement was further validated by a ~4-fold reduction in fibrosis (~18% vs ~5%, p<0.0001) seen on histology. IL10 VitaGraft increased reparative M2 macrophages 108-fold (p<0.001) and reduced Cytotoxic T cells 36% on CyTOF. This was confirmed with scRNAseq and demonstrated downregulation of inflammatory genes including the IFN-g pathway. Lastly, we demonstrated the VitaGraft could be delivered percutaneously in a pig cadaver using a pericardial catheter.
Conclusions:
Local delivery of IL10 to ischemic myocardium increases anti-inflammatory macrophages and increases reparative phenotypes. This results in improved ventricular function and reduced fibrosis after MI. The VitaGraft offers a bioengineering cell-based platform to deliver local cytokine therapy that can be inserted minimally invasively.
More abstracts on this topic:
ACS-Specific Gut Microbial and Metabolic Profiles Reveal Diagnostic and Recovery Markers
Xu Jing, Fu Jingyuan, Dai Die, Yang Yanan, Yang Jingang, Gao Shanshan, Wu Chongming, He Jiumin, Chen Weihua, Yang Yue-jin
High Throughput 3D-Printed Human Engineered Heart Tissues for Cardiac Disease ModelingJuarros Miranda, Dhand Abhishek, Martin Thomas, Valle-ayala Henry, Hunt Dakota, Burdick Jason, Leinwand Leslie