Final ID: Sa2070
CHAPERONE: Cardiovascular Kidney and Metabolic (CKM) Health Assessment and Patient Empowerment in chROnic Disease using a Health Coach iNtervention ModEl: A Randomized Clinical Trial Design Authors: Krishnaswami Vijayaraghavan, Navin Govind, Pat Dunn, Zaki Lababidi, Shishir Murarka, Sun Jones, Kevin Shah, Devendra Wadwekar, Marcus Stahlberg, Edgar Lerma, Dinesh Kalra, Keyvan Koochek, Lakshmi Nair, Samer Ibrahim, Jordi Livi, and Randal Schulhauser
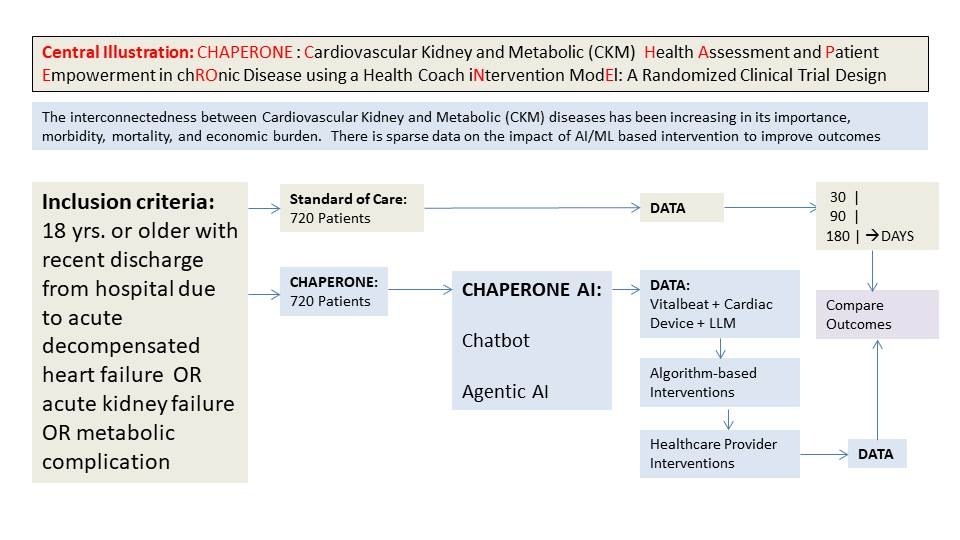
Abstract Body (Do not enter title and authors here): Background: The interconnectedness between Cardiovascular Kidney and Metabolic(CKM) diseases has been increasing in its importance, morbidity, mortality, and economic burden. There is sparse data on the impact of AI/ML based intervention to improve outcomes
Methods: This is a randomized controlled study to assess safety and efficacy of CHAPERONE Device based intervention compared with standard of care. Inclusion criteria: 18 yrs. or older with recent discharge from hospital due to acute decompensated heart failure or acute kidney failure or metabolic complication. Enrollment at 10 sites over 3 years. Randomization to SOC or Intervention arm (to receive the CHAPERONE Kit that includes monitoring and tracking of demographics cBP, blood tests ,CGM, 6 lead EKG, fluid status, CO, SVR, TFC, and HRV.) Treatment target to an A1c of 6.0, LDL <50, SBP 110- 130mm Hg, Triglyceride <150, eGFR >15, BMI < 25 and GDMT. Primary Outcome is to assess the efficacy and safety of patient empowerment, outcomes of CHF readmissions, worsening kidney disease, diabetes complications mortality through 30 days,90 days and 180 days. Secondary outcomes include LE-8 score, modified PREVENT score, Wellbeing score, KCCQ, NYHA class and technology enabled score. Sample size of 1444 subjects will be enrolled to have approximately 90% power and an alpha value of 0.05 to detect 28 % between the two groups. The primary endpoint will be a WIN RATIO based on magnitude and hierarchy of hard events to QOL
Results: Study is IRB approved. Preliminary validation of the chatbot and copilot has been performed and revealed a high level of correlation between HCP and AI based technology. Number of study subjects at the start and end of study will be reported along with demographics, number of subjects randomized, and number analyzed, frequency of the primary outcome and Win Ratio for each arm, along with 95% confidence interval and p values. All secondary and tertiary endpoints will be reported
Conclusions: The message coming out of this project will involve Best Patient outcomes in the CKM space with intensive management using user friendly technology to add value to healthcare provider as an additional tool to be utilized at low cost. This project will be able to add capacity and efficiency across multiple health systems in multiple states while providing novelty, clinical impact, sustainability , intuitiveness and accomplishing our goals to reduce disparities in care as it pertains to SDOH
Methods: This is a randomized controlled study to assess safety and efficacy of CHAPERONE Device based intervention compared with standard of care. Inclusion criteria: 18 yrs. or older with recent discharge from hospital due to acute decompensated heart failure or acute kidney failure or metabolic complication. Enrollment at 10 sites over 3 years. Randomization to SOC or Intervention arm (to receive the CHAPERONE Kit that includes monitoring and tracking of demographics cBP, blood tests ,CGM, 6 lead EKG, fluid status, CO, SVR, TFC, and HRV.) Treatment target to an A1c of 6.0, LDL <50, SBP 110- 130mm Hg, Triglyceride <150, eGFR >15, BMI < 25 and GDMT. Primary Outcome is to assess the efficacy and safety of patient empowerment, outcomes of CHF readmissions, worsening kidney disease, diabetes complications mortality through 30 days,90 days and 180 days. Secondary outcomes include LE-8 score, modified PREVENT score, Wellbeing score, KCCQ, NYHA class and technology enabled score. Sample size of 1444 subjects will be enrolled to have approximately 90% power and an alpha value of 0.05 to detect 28 % between the two groups. The primary endpoint will be a WIN RATIO based on magnitude and hierarchy of hard events to QOL
Results: Study is IRB approved. Preliminary validation of the chatbot and copilot has been performed and revealed a high level of correlation between HCP and AI based technology. Number of study subjects at the start and end of study will be reported along with demographics, number of subjects randomized, and number analyzed, frequency of the primary outcome and Win Ratio for each arm, along with 95% confidence interval and p values. All secondary and tertiary endpoints will be reported
Conclusions: The message coming out of this project will involve Best Patient outcomes in the CKM space with intensive management using user friendly technology to add value to healthcare provider as an additional tool to be utilized at low cost. This project will be able to add capacity and efficiency across multiple health systems in multiple states while providing novelty, clinical impact, sustainability , intuitiveness and accomplishing our goals to reduce disparities in care as it pertains to SDOH
More abstracts on this topic:
A Randomized Comparison of Online Motivational Themes in Cardiovascular Clinical Trial Recruitment
Hussain Zaib, Harry Tamunotonye, Michos Erin, Milller Hailey, Juraschek Stephen, Turkson-ocran Ruth-alma, Lahey Timothy, Feng Yuanyuan, Plante Timothy
A Polypill Strategy for Heart Failure with Reduced Ejection Fraction: The POLY-HF TrialPandey Ambarish, Wang Thomas, Keshvani Neil, Rizvi Syed Kazim, Jain Anand, Coellar Juan David, Drazner Mark, Gupta Deepak, Chandra Alvin, Zaha Vlad