Final ID: 4371138
Polygenic Risk Score–Based Enrichment of Primary Prevention Cohorts for Atherosclerotic Cardiovascular Disease Trials
Abstract Body (Do not enter title and authors here): Background: Clinical trials for coronary artery disease (CAD) face significant challenges in cost, duration, and participant recruitment, especially in primary prevention settings. Enrichment strategies aim to improve trial efficiency; prognostic enrichment specifically selects participants at higher baseline risk of disease events. Polygenic risk scores (PRS) offer a potential tool for identifying such individuals based on their genetic predisposition.
Research Question: Evaluation of the utility of a CAD PRS for prognostic enrichment of hypothetical phase 3 primary prevention cardiovascular outcome trials using real-world data from the Mass General Brigham (MGB) healthcare system and establish proof-of-concept for trial design methodology.
Methods: A primary prevention cohort (N=16,569) of adults aged ≥30 without previous atherosclerotic cardiovascular disease (ASCVD) was identified from the MGB Biobank linked to electronic health records. Individual CAD PRS were calculated, defining a high-risk group (≥80th percentile PRS). The standard Pooled Cohort Equations (PCE) risk was compared to a PRS-adjusted PCE risk stratification for ASCVD. We ran individual-level Monte Carlo microsimulations to simulate trials to compare the effect of 3 recruitment strategies: No PRS enrichment, hybrid enrichment (50% from the high-risk PRS group / 50% other), and exclusively high-PRS risk group. Outcomes included time-to-target event accrual, power at fixed time, and required sample size.
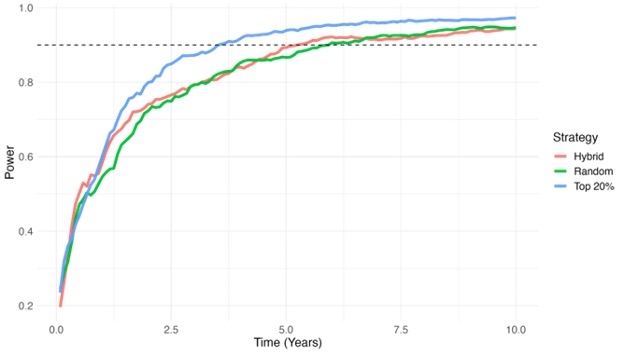
Results: The mean age was 58 ± 14.7 years. PRS effectively stratified ASCVD risk and significantly reclassified risk compared to PCE alone (17% upward,15.4% downward,and 1.6% net upward reclassification). Trial simulations demonstrated substantial efficiency gains with PRS-based prognostic enrichment. Compared to no PRS enrichment, recruiting only the high-risk PRS group reduced time-to-90%-power by approximately 2.4 years (Figure), increased power over 3 years (0.87 vs. 0.80), and reduced the required sample size by ~25% (8,109 vs. 10,833) to achieve 90% with 3 years of follow-up.
Conclusion: This study provides a strong proof-of-concept that CAD PRS-based prognostic enrichment can significantly improve the efficiency of primary prevention cardiovascular trials, offering potential reductions in trial duration and sample size. While promising, prospective validation is required, alongside careful consideration of generalizability and equity across diverse populations.
Research Question: Evaluation of the utility of a CAD PRS for prognostic enrichment of hypothetical phase 3 primary prevention cardiovascular outcome trials using real-world data from the Mass General Brigham (MGB) healthcare system and establish proof-of-concept for trial design methodology.
Methods: A primary prevention cohort (N=16,569) of adults aged ≥30 without previous atherosclerotic cardiovascular disease (ASCVD) was identified from the MGB Biobank linked to electronic health records. Individual CAD PRS were calculated, defining a high-risk group (≥80th percentile PRS). The standard Pooled Cohort Equations (PCE) risk was compared to a PRS-adjusted PCE risk stratification for ASCVD. We ran individual-level Monte Carlo microsimulations to simulate trials to compare the effect of 3 recruitment strategies: No PRS enrichment, hybrid enrichment (50% from the high-risk PRS group / 50% other), and exclusively high-PRS risk group. Outcomes included time-to-target event accrual, power at fixed time, and required sample size.
Results: The mean age was 58 ± 14.7 years. PRS effectively stratified ASCVD risk and significantly reclassified risk compared to PCE alone (17% upward,15.4% downward,and 1.6% net upward reclassification). Trial simulations demonstrated substantial efficiency gains with PRS-based prognostic enrichment. Compared to no PRS enrichment, recruiting only the high-risk PRS group reduced time-to-90%-power by approximately 2.4 years (Figure), increased power over 3 years (0.87 vs. 0.80), and reduced the required sample size by ~25% (8,109 vs. 10,833) to achieve 90% with 3 years of follow-up.
Conclusion: This study provides a strong proof-of-concept that CAD PRS-based prognostic enrichment can significantly improve the efficiency of primary prevention cardiovascular trials, offering potential reductions in trial duration and sample size. While promising, prospective validation is required, alongside careful consideration of generalizability and equity across diverse populations.
More abstracts on this topic:
A multi-task deep learning algorithm for detecting obstructive coronary artery disease using fundus photographs
Zeng Yong, Ding Yaodong
Combining Noncoding and Coding Genetic Variation Improves Arrhythmia Risk PredictionMonroe Tanner, Pesce Lorenzo, Kearns Samuel, Page Patrick, Dellefave-castillo Lisa, Webster Gregory, Puckelwartz Megan, Mcnally Elizabeth