Final ID: MP2227
Prognosis of Cardiovascular-Kidney-Metabolic (CKM) Stages: The Dallas Heart Study
Abstract Body (Do not enter title and authors here): Background: The recent AHA presidential advisory on Cardiovascular-Kidney-Metabolic Syndrome (CKM) proposed a novel staging scheme, but limited data exist regarding the associations of CKM Stages with incident cardiovascular events and mortality.
Methods: We included participants in the Dallas Heart Study, a population-sampled cohort from Dallas County, who attended study Visit 1 (2000-2002) and underwent protocol measurement of body composition, lipids, fasting blood sugar, serum creatinine, NT-proBNP, hs-cTnT, urinary albumin and creatinine, coronary artery calcium by cardiac CT (CAC), and cardiac function and mass by cardiac MRI. CKM Stages were defined as per AHA CKM definitions: Stage 0 – no CKM risk factors; 1 – excess or dysfunctional adiposity; 2 – metabolic risk factors and/or chronic kidney disease; 3 – subclinical cardiovascular diseases [CAC, left ventricular hypertrophy or dysfunction by cardiac MRI, elevated cardiac biomarkers (NT-proBNP or hs-cTnT), high AHA-PREVENT or KDIGO scores]; 4 – prevalent cardiovascular diseases [coronary heart disease (CHD), heart failure (HF), atrial fibrillation, stroke]. Participants were followed for fatal and non-fatal clinical outcomes, including CHD, HF, and stroke through December 31th 2018. Multivariable Cox proportional hazard models were used to assess the relationship of the CKM Stage with incident events compared to absent CKM or Stage 1, adjusting for age, sex, and race.
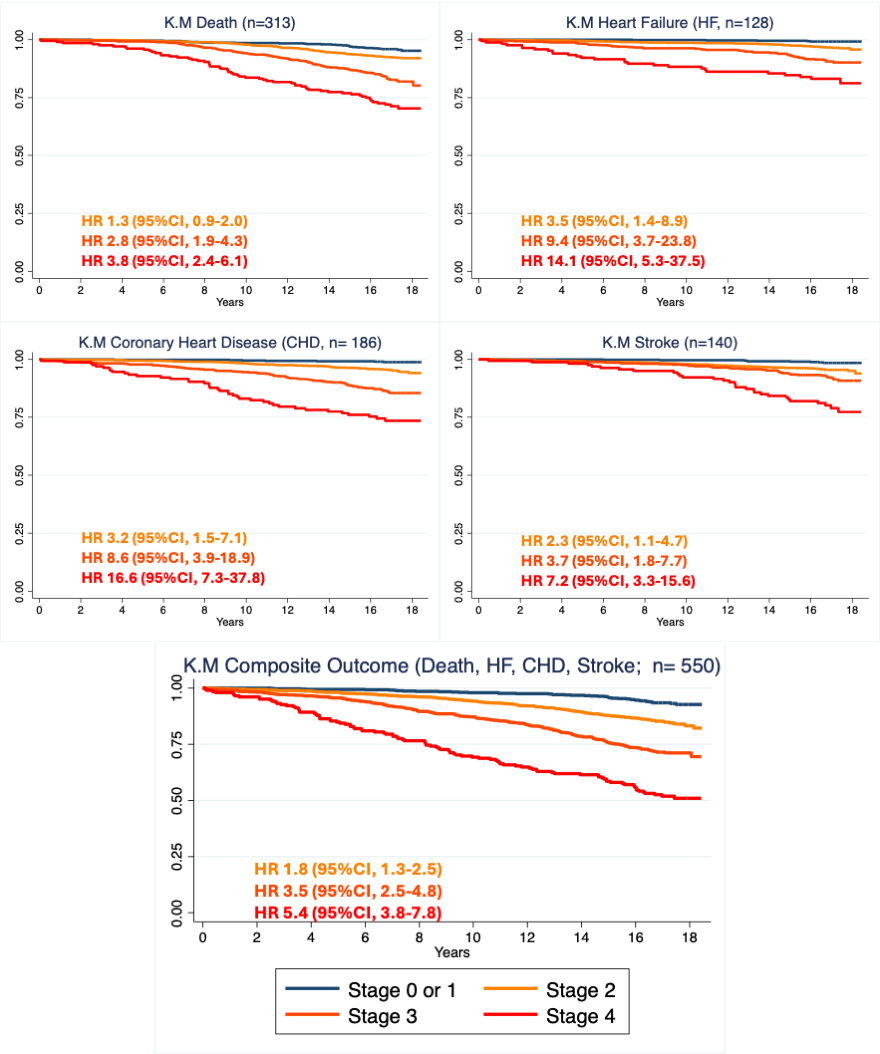
Results: Among the 2,991 participants (age 44±10 years, 56% female, 50% reported non-Hispanic Black race), CKM stage prevalence was 9% Stage 0, 14% Stage 1, 47% Stage 2, 22% Stage 3, and 7% Stage 4. Over a median follow-up of 16.9 (IQR 16.4 -17.6) years, 19% died or developed CHD, HF, or stroke. No significant differences in risk were observed between Stage 0 and Stage 1 for composite and individual outcomes. Compared to those with Stage 0 or 1 CKM, a graded association was observed between greater CKM Stage 2-4 and heightened risk of composite CHD, HF, stroke, or death [HR 1.8 (95% CI 1.3-2.5), 3.5 (2.5-4.8), 5.4 (3.8-7.8), respectively] in adjusted model. Similar trends were observed for each component of the composite (Figure).
Conclusion: Over 17 years of follow-up, individuals with CKM Stage 1 did not experience worse outcomes than those free of CKM Stage. CKM Stages 2, 3, and 4 were associated with a stepwise higher risk of all cause mortality and incident CHD, HF, and stroke compared to those with Stage 0 or 1 CKM.
Methods: We included participants in the Dallas Heart Study, a population-sampled cohort from Dallas County, who attended study Visit 1 (2000-2002) and underwent protocol measurement of body composition, lipids, fasting blood sugar, serum creatinine, NT-proBNP, hs-cTnT, urinary albumin and creatinine, coronary artery calcium by cardiac CT (CAC), and cardiac function and mass by cardiac MRI. CKM Stages were defined as per AHA CKM definitions: Stage 0 – no CKM risk factors; 1 – excess or dysfunctional adiposity; 2 – metabolic risk factors and/or chronic kidney disease; 3 – subclinical cardiovascular diseases [CAC, left ventricular hypertrophy or dysfunction by cardiac MRI, elevated cardiac biomarkers (NT-proBNP or hs-cTnT), high AHA-PREVENT or KDIGO scores]; 4 – prevalent cardiovascular diseases [coronary heart disease (CHD), heart failure (HF), atrial fibrillation, stroke]. Participants were followed for fatal and non-fatal clinical outcomes, including CHD, HF, and stroke through December 31th 2018. Multivariable Cox proportional hazard models were used to assess the relationship of the CKM Stage with incident events compared to absent CKM or Stage 1, adjusting for age, sex, and race.
Results: Among the 2,991 participants (age 44±10 years, 56% female, 50% reported non-Hispanic Black race), CKM stage prevalence was 9% Stage 0, 14% Stage 1, 47% Stage 2, 22% Stage 3, and 7% Stage 4. Over a median follow-up of 16.9 (IQR 16.4 -17.6) years, 19% died or developed CHD, HF, or stroke. No significant differences in risk were observed between Stage 0 and Stage 1 for composite and individual outcomes. Compared to those with Stage 0 or 1 CKM, a graded association was observed between greater CKM Stage 2-4 and heightened risk of composite CHD, HF, stroke, or death [HR 1.8 (95% CI 1.3-2.5), 3.5 (2.5-4.8), 5.4 (3.8-7.8), respectively] in adjusted model. Similar trends were observed for each component of the composite (Figure).
Conclusion: Over 17 years of follow-up, individuals with CKM Stage 1 did not experience worse outcomes than those free of CKM Stage. CKM Stages 2, 3, and 4 were associated with a stepwise higher risk of all cause mortality and incident CHD, HF, and stroke compared to those with Stage 0 or 1 CKM.
More abstracts on this topic:
ALDH2 rs671 polymorphism and metabolic signatures in patients with cardiovascular diseases
Zeng Linqi
A Trial of Patients Receiving Remote Ischemic Conditioning in Early Stroke (PRICES) in a Tertiary Hospital in the Philippines: An Open Label StudyAng Kevin Royce, Juangco Dan, Hernandez Maria Kim