Final ID: MP2745
Metabolomic Profiling in a Murine Model of HFpEF Reveals Fuel Metabolism Alterations and Accumulation of Gut-Derived Metabolites
Abstract Body (Do not enter title and authors here): Background:
HFpEF is a highly prevalent yet poorly understood syndrome that lacks disease-modifying therapies. While dysregulation of fuel substrate metabolic pathways is recognized in heart failure, their role in HFpEF remains incompletely defined. We applied untargeted metabolomic profiling across multiple tissues in murine models of HFpEF and HFrEF to investigate alterations in canonical fuel metabolism and identify novel metabolic pathways relevant to disease pathogenesis.
Methods:
Male C57BL/6N mice were randomized to receive standard chow, high-fat diet plus L-NAME (HFpEF model), or transverse aortic constriction (HFrEF model). HFpEF mice were treated for 5 (short-term) or 15 (long-term) weeks. Serum, liver, and left ventricular tissues were analyzed by untargeted mass spectrometry-based metabolomics. Metabolite levels were compared between HF and standard chow groups. Canonical fuel substrate pathways—branched-chain amino acids (BCAA), fatty acid oxidation (FAO), and ketones—were analyzed alongside discovery-driven profiling.
Results:
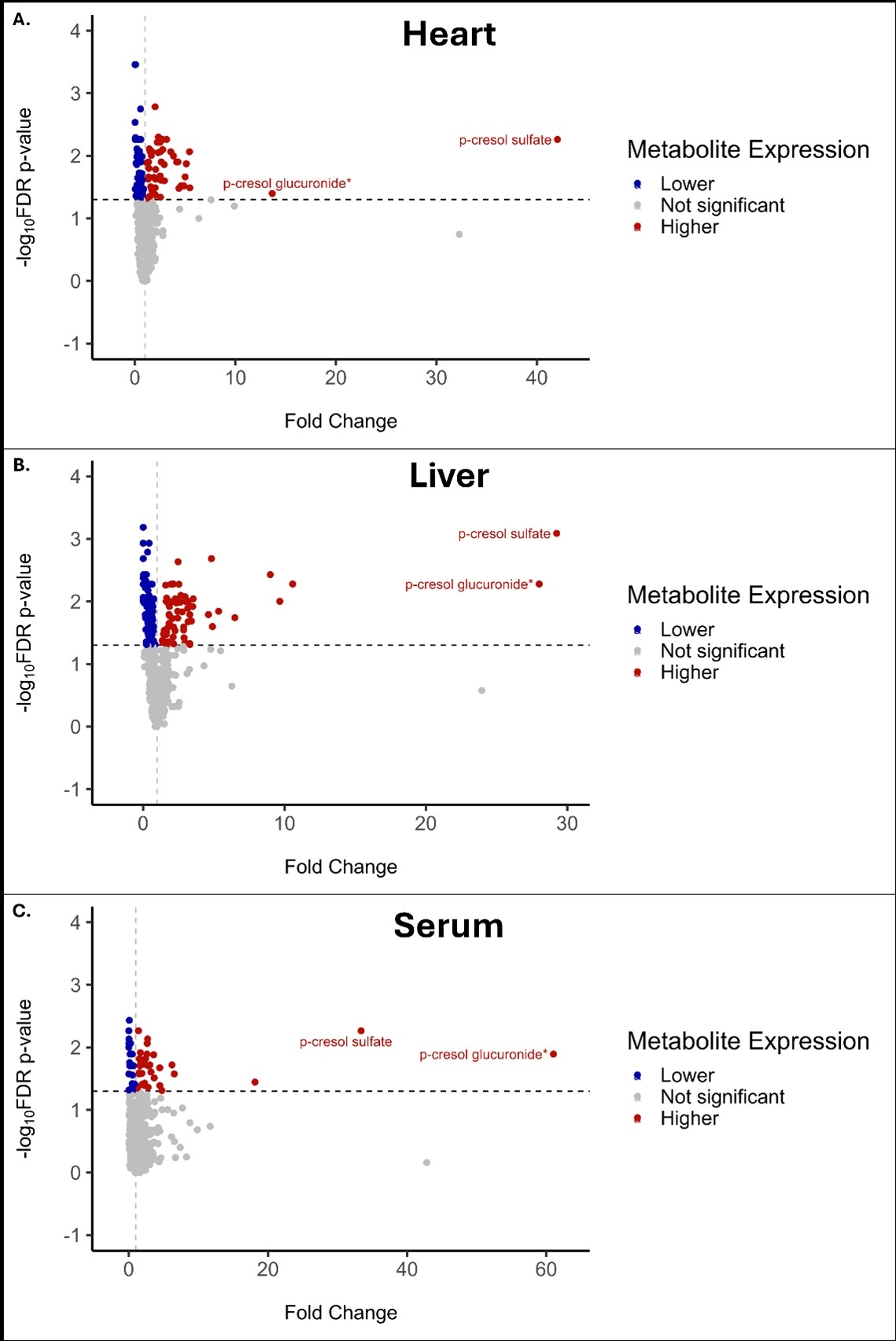
Analysis of canonical fuel substrate pathways revealed evidence of higher myocardial BCAA levels with lower downstream catabolic levels, suggesting higher BCAA uptake with a potential bottleneck at BCKDH in both HFpEF and HFrEF; reductions in myocardial FAO intermediates in HFpEF; and higher ketone body levels in heart and serum of HFpEF vs. standard chow. Discovery analysis identified p-cresol sulfate and p-cresol glucuronide— conjugated derivatives of p-cresol, a microbial metabolite of tyrosine fermentation —as the most significantly and consistently elevated metabolites in HFpEF across tissues. In the long-term model, p-cresol sulfate levels were increased by 42.0-fold in heart, 29.3-fold in liver, and 33.4-fold in serum; p-cresol glucuronide was increased by 13.7-, 28.0-, and 61.0-fold, respectively (all q < 0.05). Notably, these changes were not observed in HFrEF. Elevations in p-Cresol sulfate were corroborated in plasma from human HFpEF subjects.
Conclusions:
This tissue-wide metabolomic analysis in murine HFpEF and HFrEF models reveals HFpEF-specific metabolic remodeling—marked by impaired BCAA catabolism, reduced myocardial FAO, and enhanced ketone metabolism—mirroring human disease. The striking accumulation of microbial-derived p-cresol conjugates in HFpEF, but not HFrEF, suggests gut-liver-heart axis disruption and nominates these metabolites as candidate biomarkers or therapeutic targets.
HFpEF is a highly prevalent yet poorly understood syndrome that lacks disease-modifying therapies. While dysregulation of fuel substrate metabolic pathways is recognized in heart failure, their role in HFpEF remains incompletely defined. We applied untargeted metabolomic profiling across multiple tissues in murine models of HFpEF and HFrEF to investigate alterations in canonical fuel metabolism and identify novel metabolic pathways relevant to disease pathogenesis.
Methods:
Male C57BL/6N mice were randomized to receive standard chow, high-fat diet plus L-NAME (HFpEF model), or transverse aortic constriction (HFrEF model). HFpEF mice were treated for 5 (short-term) or 15 (long-term) weeks. Serum, liver, and left ventricular tissues were analyzed by untargeted mass spectrometry-based metabolomics. Metabolite levels were compared between HF and standard chow groups. Canonical fuel substrate pathways—branched-chain amino acids (BCAA), fatty acid oxidation (FAO), and ketones—were analyzed alongside discovery-driven profiling.
Results:
Analysis of canonical fuel substrate pathways revealed evidence of higher myocardial BCAA levels with lower downstream catabolic levels, suggesting higher BCAA uptake with a potential bottleneck at BCKDH in both HFpEF and HFrEF; reductions in myocardial FAO intermediates in HFpEF; and higher ketone body levels in heart and serum of HFpEF vs. standard chow. Discovery analysis identified p-cresol sulfate and p-cresol glucuronide— conjugated derivatives of p-cresol, a microbial metabolite of tyrosine fermentation —as the most significantly and consistently elevated metabolites in HFpEF across tissues. In the long-term model, p-cresol sulfate levels were increased by 42.0-fold in heart, 29.3-fold in liver, and 33.4-fold in serum; p-cresol glucuronide was increased by 13.7-, 28.0-, and 61.0-fold, respectively (all q < 0.05). Notably, these changes were not observed in HFrEF. Elevations in p-Cresol sulfate were corroborated in plasma from human HFpEF subjects.
Conclusions:
This tissue-wide metabolomic analysis in murine HFpEF and HFrEF models reveals HFpEF-specific metabolic remodeling—marked by impaired BCAA catabolism, reduced myocardial FAO, and enhanced ketone metabolism—mirroring human disease. The striking accumulation of microbial-derived p-cresol conjugates in HFpEF, but not HFrEF, suggests gut-liver-heart axis disruption and nominates these metabolites as candidate biomarkers or therapeutic targets.
More abstracts on this topic:
9-Year Longitudinal Assessment of the 12-lead Electrocardiogram of Volunteer Firefighters
Bae Alexander, Dzikowicz Dillon, Lai Chi-ju, Brunner Wendy, Krupa Nicole, Carey Mary, Tam Wai Cheong, Yu Yichen
4-Hydroxy-2-Nonenal Alters Alternative Polyadenylation to Regulate mRNA Isoform Diversity in the Transition from Human Cardiac Fibroblasts to MyofibroblastsNatarajan Kartiga, Neupane Rahul, Yalamanchili Hari Krishna, Palaniyandi Suresh, Wagner Eric, Guha Ashrith, Amirthalingam Thandavarayan Rajarajan