Final ID: MP1785
Real-World Adverse Events of the Aurora Extravascular Implantable Cardiac Defibrillator: Insights from the United States FDA MAUDE Registry
Abstract Body (Do not enter title and authors here): Introduction:
Extravascular implantable cardioverter defibrillators (EV-ICD) utilize a substernal lead to provide the benefits of ICDs without associated vascular risks. Only a few currently exist, and one such is the Aurora EV-ICDTM (Medtronic, Minneapolis, MN). Device-related, real-world complication data remains limited.
Objectives:
We reviewed the United States Food and Drug Manufacturer and User Facility Device Experience (MAUDE) database for adverse events involving the Aurora EV-ICD.
Methods:
The MAUDE database is a real-world registry of device-related adverse events. We reviewed this on 6/4/2025 for all events since 10/1/2023 – shortly after it was approved for use. Duplicate reports were removed, and adverse events and device issues were categorized and analyzed.
Results:
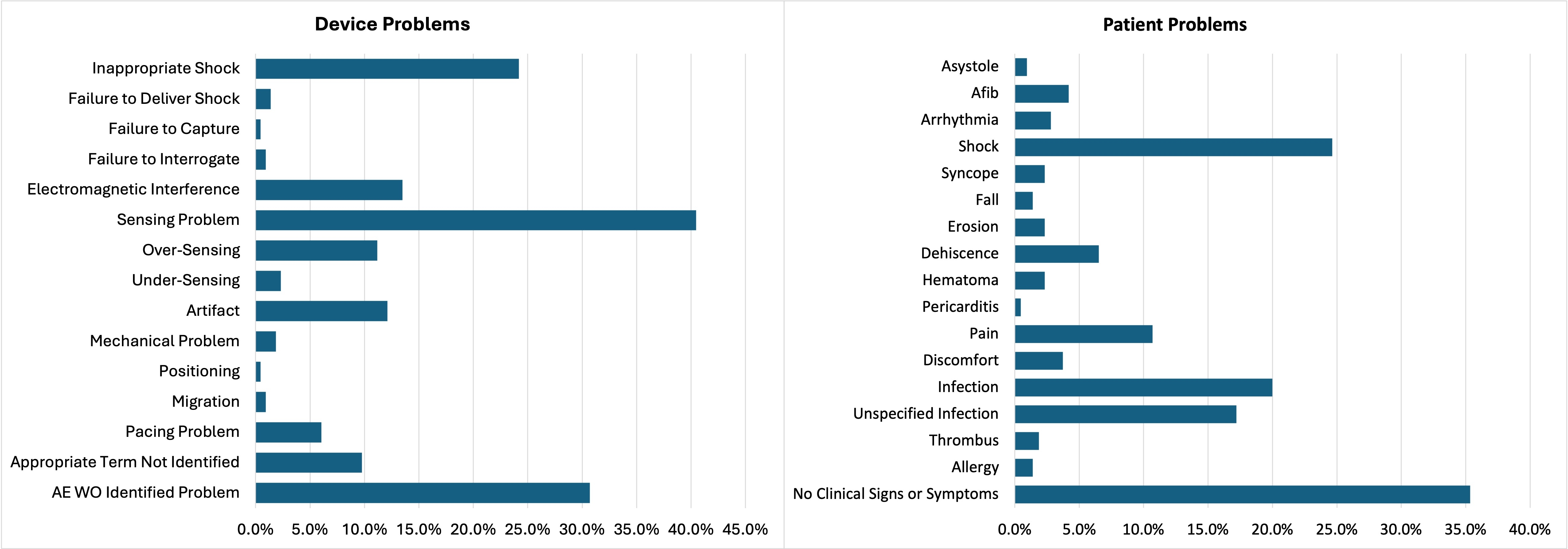
A total of 215 reports were identified, with an average age of 48.3±16.8 years and 75.0% male gender. Adverse events were analyzed as device- and patient-related. The most common device problem was sensing issues (40.4%), which included over- (11.1%) and under-sensing (2.3%); adverse events without an identified problem also occurred frequently (30.7%). The most common patient events occurred without clinical signs or symptoms (35.3%) but were followed by infection (37.3%) and inappropriate shocks (24.7%). This resulted in injury in 77.7% of cases, with death occurring in rare (1.9%) cases.
Conclusion:
EV-ICDs are an alternative defibrillator option but have shown notable early challenges, including frequent sensing issues, inappropriate shocks, and a high rate of infections. These findings underscore the need for careful patient selection and follow-up. However, the reliance on the MAUDE limits generalizability, and further studies are needed to evaluate its safety and efficacy.
Extravascular implantable cardioverter defibrillators (EV-ICD) utilize a substernal lead to provide the benefits of ICDs without associated vascular risks. Only a few currently exist, and one such is the Aurora EV-ICDTM (Medtronic, Minneapolis, MN). Device-related, real-world complication data remains limited.
Objectives:
We reviewed the United States Food and Drug Manufacturer and User Facility Device Experience (MAUDE) database for adverse events involving the Aurora EV-ICD.
Methods:
The MAUDE database is a real-world registry of device-related adverse events. We reviewed this on 6/4/2025 for all events since 10/1/2023 – shortly after it was approved for use. Duplicate reports were removed, and adverse events and device issues were categorized and analyzed.
Results:
A total of 215 reports were identified, with an average age of 48.3±16.8 years and 75.0% male gender. Adverse events were analyzed as device- and patient-related. The most common device problem was sensing issues (40.4%), which included over- (11.1%) and under-sensing (2.3%); adverse events without an identified problem also occurred frequently (30.7%). The most common patient events occurred without clinical signs or symptoms (35.3%) but were followed by infection (37.3%) and inappropriate shocks (24.7%). This resulted in injury in 77.7% of cases, with death occurring in rare (1.9%) cases.
Conclusion:
EV-ICDs are an alternative defibrillator option but have shown notable early challenges, including frequent sensing issues, inappropriate shocks, and a high rate of infections. These findings underscore the need for careful patient selection and follow-up. However, the reliance on the MAUDE limits generalizability, and further studies are needed to evaluate its safety and efficacy.
More abstracts on this topic:
Adverse Clinical Events Due to the Safety Mode in Implantable Defibrillators
Desouki Mariam, Abdelsayed Kerollos, Witt Dawn, Sengupta Jay, Hauser Robert
Arrhythmic Risk Stratification of Patients with Suspected Cardiac Sarcoidosis, High-Grade Atrioventricular Block, and No Late Gadolinium Enhancement on Cardiovascular Magnetic Resonance Imaging: A Multicenter StudyBawaskar Parag, De Leeuw Beverly, Rochlani Yogita, Mathijssen Harold, Markowitz Jeremy, Von Wald Lisa, Roukoz Henri, Post Marco, Shenoy Chetan