Final ID: MP1193
Polygenic Risk-Based Detection and Treatment of Subclinical Coronary Atherosclerosis in the PROACT Clinical Trials
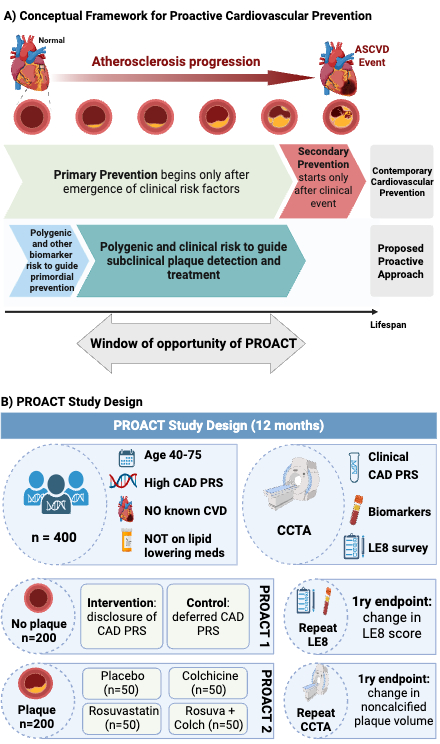
Methods: Adults aged 40-75 years without cardiovascular disease or lipid-lowering therapy but high CAD PRS undergo cardiovascular health evaluations and coronary computed tomography angiography (CCTA). Participants without plaque are enrolled in PROACT 1, a randomized trial (NCT05819814) evaluating the impact of high genetic risk disclosure vs. standard care on 12-month change in cardiovascular health, measured by the American Heart Association Life’s Essential 8 (LE8) score. Participants with quantifiable plaque are enrolled in PROACT 2, a double-blind, 4-arm randomized trial (NCT05850091) assessing rosuvastatin 20 mg and/or colchicine 0.6 mg vs. placebo on 12 month change in non-calcified plaque volume (Fig 1B).
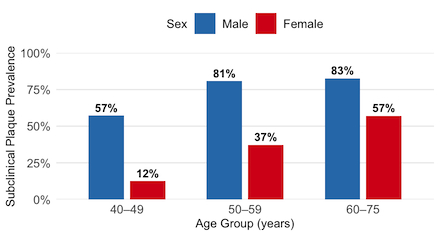
Results: Among 64,092 genotyped Mass General Brigham Biobank participants, 2,495 were eligible and had high CAD PRS despite low clinical risk – median 10-year Pooled Cohort Equations ASCVD risk 3% (IQR 1-8). Recruitment shows high engagement–among 1,314 invited individuals, 283 (21.5%) expressed interest. Analysis of the first 204 participants enrolled by January 31st, 2025 (mean age 55.7±8.6 years; 69% female) show that despite the low clinical risk and better cardiovascular health (mean LE8 73.3±11.5 compared to the US average of ~65), half the participants (102 of 204) had subclinical plaque. Subclinical plaque prevalence was high across sexes and age groups (Fig 2). Conventional clinical risk factors were not significant predictors of plaque in this low-clinical, high-genetic risk group.
Conclusions: Clinical risk-factor based models miss a substantial “silent” population with high genetic risk and subclinical coronary plaque. PROACT demonstrates that (1) these individuals are receptive to genetic-enriched plaque detection and (2) half already have subclinical coronary plaque despite low clinical risk. Ongoing randomized interventions in PROACT 1 and PROACT 2 will determine whether PRS disclosure and pharmacological interventions can improve cardiovascular health and regress subclinical coronary atherosclerosis.
- Abou-karam, Roukoz ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Ellinor, Patrick ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Foldyna, Borek ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Ghebremichael, Musie ( Ragon Institute of MGH, MIT, and Harvard , Cambridge , Massachusetts , United States )
- Atlas, Steven ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Ridker, Paul ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Lu, Michael ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Fahed, Akl ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Kim, Min Seo ( Broad Institute of MIT and Harvard , Cambridge , Massachusetts , United States )
- Cho, So Mi ( Broad Institute of MIT and Harvard , Cambridge , Massachusetts , United States )
- Bitar, Fouad ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Gady, Shoshana ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Cheng, Fangzhou ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Thompson, Abigail Grace ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Karlson, Elizabeth ( Mass General Brigham , Boston , Massachusetts , United States )
- Natarajan, Pradeep ( Massachusetts General Hospital , Boston , Massachusetts , United States )
Meeting Info:
Session Info:
Multi-Omic Insights into Coronary Artery Disease 1
Saturday, 11/08/2025 , 03:15PM - 04:15PM
Moderated Digital Poster Session
More abstracts on this topic:
Avgousti Harris, Johnson Ethan, Berhane Haben, Thomas James, Allen Bradley, Markl Michael, Appadurai Vinesh
Adding a polygenic risk score to the PREVENT clinical risk tool significantly improves cardiovascular risk predictionEuesden Jack, Absher Devin, Iribarren Carlos, Riveros-mckay Fernando, Rana Jamal, Rowell Sarah, Neogi Arpita, Harrison Seamus, Weale Michael, Donnelly Peter
More abstracts from these authors:
Cho So Mi, Natarajan Pradeep, Rivera Rachel, Koyama Satoshi, Kim Min Seo, Honigberg Michael, Bhattacharya Romit, Paruchuri Kaavya, Allen Norrina, Hornsby Whitney
Diagnostic Yield and Testing Characteristics of an Invasive Coronary Function Testing ProgramBitar Fouad, Abou-karam Roukoz, Cheng Fangzhou, Gady Shoshana, Sakhuja Rahul, Jaffer Farouc, Fahed Akl