Final ID: MP2038
AAV-mediated Gene Delivery of PERM1 Prevents the Development of Heart Failure with Reduced Ejection Fraction in a Mouse Model of Pressure Overload
Abstract Body (Do not enter title and authors here): Introduction: Heart failure with reduced ejection fraction (HFrEF) affects over 3 million adults in the United States with high mortality. HFrEF is marked by impaired cardiomyocyte contractility and disrupted energy metabolism; however, no current therapies target both. Our recent study showed that adeno-associated virus (AAV)-mediated overexpression of PERM1, a striated muscle-specific regulator of mitochondrial bioenergetics, enhances both cardiac contractility and mitochondrial biogenesis in healthy mouse hearts. Whether AAV-PERM1 can be therapeutic in HFrEF remains unknown.
Hypothesis: AAV-mediated gene delivery of PERM1 mitigates HFrEF onset by simultaneously preserving mitochondrial biogenesis and cardiac contractility under pressure overload.
Methods: To test the cardioprotective effects of AAV-PERM1 during pathological stress, 8-12-week-old wild-type C57BL/6 mice were treated with either AAV9-PERM1 or control AAV9-GFP (1×1012 GC/mouse), followed by transverse aortic constriction (TAC) surgery (Fig.1A).
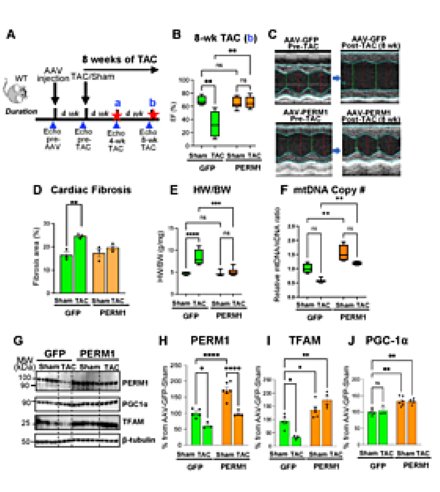
Results: At 4 weeks post-TAC, AAV-GFP mice exhibited reduced left ventricular ejection fraction (LVEF) (64% in sham vs. 32% in TAC, p<0.05). In contrast, AAV-PERM1 preserved LVEF post-TAC (68% in sham vs. 67% in TAC, p=0.904). This protective effect was sustained through 8 weeks (Fig.1B-C) without TAC-induced fibrosis (16.5% in AAV-GFP-Sham vs. 19.7% in AAV-PERM1-TAC, p>0.05, Fig.1D). TAC-induced hypertrophy, reflected in an increased heart weight-to-body weight ratio, was blunted by AAV-PERM1 (8.3 vs. 5.1 in AAV-GFP-TAC vs. AAV-PERM1-TAC, p<0.05; Fig.1E). Mitochondrial DNA copy number was reduced by TAC in AAV-GFP mice (0.6-fold vs. sham, p<0.05), which was fully preserved with AAV-PERM1 (1.2-fold vs. AAV-GFP-sham, p>0.05; Fig.1F), consistent with maintained TFAM protein levels (Fig.1G,I). PGC-1α expression was unchanged by TAC in AAV-GFP mice (p>0.05) but was modestly yet significantly increased by AAV-PERM1 in both sham and TAC hearts (both p<0.05 vs. GFP sham; Fig.1G,J). Lastly, western blot analysis confirmed effective gene delivery of PERM1. While PERM1 protein levels were reduced by TAC (61% of GFP-sham, p<0.05), they were fully maintained with AAV-PERM1 (99% of GFP-sham, p>0.05; Fig.1G-H).
Conclusions: AAV-PERM1 effectively prevents pathological hypertrophy with fully preserved mitochondrial biogenesis under pressure overload, demonstrating a multifaceted gene therapy for HFrEF that targets contractile dysfunction and mitochondrial impairment.
Hypothesis: AAV-mediated gene delivery of PERM1 mitigates HFrEF onset by simultaneously preserving mitochondrial biogenesis and cardiac contractility under pressure overload.
Methods: To test the cardioprotective effects of AAV-PERM1 during pathological stress, 8-12-week-old wild-type C57BL/6 mice were treated with either AAV9-PERM1 or control AAV9-GFP (1×1012 GC/mouse), followed by transverse aortic constriction (TAC) surgery (Fig.1A).
Results: At 4 weeks post-TAC, AAV-GFP mice exhibited reduced left ventricular ejection fraction (LVEF) (64% in sham vs. 32% in TAC, p<0.05). In contrast, AAV-PERM1 preserved LVEF post-TAC (68% in sham vs. 67% in TAC, p=0.904). This protective effect was sustained through 8 weeks (Fig.1B-C) without TAC-induced fibrosis (16.5% in AAV-GFP-Sham vs. 19.7% in AAV-PERM1-TAC, p>0.05, Fig.1D). TAC-induced hypertrophy, reflected in an increased heart weight-to-body weight ratio, was blunted by AAV-PERM1 (8.3 vs. 5.1 in AAV-GFP-TAC vs. AAV-PERM1-TAC, p<0.05; Fig.1E). Mitochondrial DNA copy number was reduced by TAC in AAV-GFP mice (0.6-fold vs. sham, p<0.05), which was fully preserved with AAV-PERM1 (1.2-fold vs. AAV-GFP-sham, p>0.05; Fig.1F), consistent with maintained TFAM protein levels (Fig.1G,I). PGC-1α expression was unchanged by TAC in AAV-GFP mice (p>0.05) but was modestly yet significantly increased by AAV-PERM1 in both sham and TAC hearts (both p<0.05 vs. GFP sham; Fig.1G,J). Lastly, western blot analysis confirmed effective gene delivery of PERM1. While PERM1 protein levels were reduced by TAC (61% of GFP-sham, p<0.05), they were fully maintained with AAV-PERM1 (99% of GFP-sham, p>0.05; Fig.1G-H).
Conclusions: AAV-PERM1 effectively prevents pathological hypertrophy with fully preserved mitochondrial biogenesis under pressure overload, demonstrating a multifaceted gene therapy for HFrEF that targets contractile dysfunction and mitochondrial impairment.
More abstracts on this topic:
A Case of Dilated Cardiomyopathy and Systemic Thromboembolism in a Young Patient on Testosterone Replacement Therapy
Sabri Muhammad, Ijaz Naila, Nadeem Ramsha, Checchio Lucy, Riaz Faiza
β1 Adrenergic Receptor Autoantibodies Promote Heart Failure Though Activation of Prostaglandin E2 Receptor EP1/Phosphodiesterase 4B PathwayCao Ning, Qiu Hui, Li Hongwei