Final ID: 4367722
Mitochondrial Transplantation During Ongoing Ischemia May Preserve Neuronal Viability
Abstract Body (Do not enter title and authors here): Introduction: Cardiac and aortic surgery carries a relatively high risk of perioperative stroke. Thrombolysis and thrombectomy are often not feasible in the perioperative setting for these patients. While restoring cerebral blood flow is critical, it can trigger reperfusion injury. Ischemia-reperfusion (IR) injury disrupts mitochondrial function and can lead to neuronal cell death. Post-ischemic mitochondrial transplantation (MTx) has shown neuroprotection in preclinical studies, but its efficacy during ischemia remains unclear. Additionally, nicorandil (NIC), a mitochondrial KATP channel opener, can improve metabolic tolerance during IR injury.
Hypothesis/Aims: This study aimed to evaluate whether MTx during ongoing ischemia preserves neuronal viability and mitochondrial function in an in vitro oxygen-glucose deprivation (OGD) model, and whether NIC co-treatment provides a synergistic effect.
Methods: The study included two main groups: normoxic control and OGD; each had 9 subgroups: no treatment, 10 or 0.1µg/mL mitochondria (Mito), 100 or 500µM NIC, and their combinations. HT-22 neuronal cells were subjected to OGD for 10h, with Mito and/or NIC added 4h after initiation. Controls remained under normoxia. After OGD, all subgroups returned to normoxia for 17h. Mitochondria were isolated from mouse skeletal muscle. Cell viability, proliferation, and mitochondrial function were analyzed using MTS, CyQUANT, and Seahorse assays.
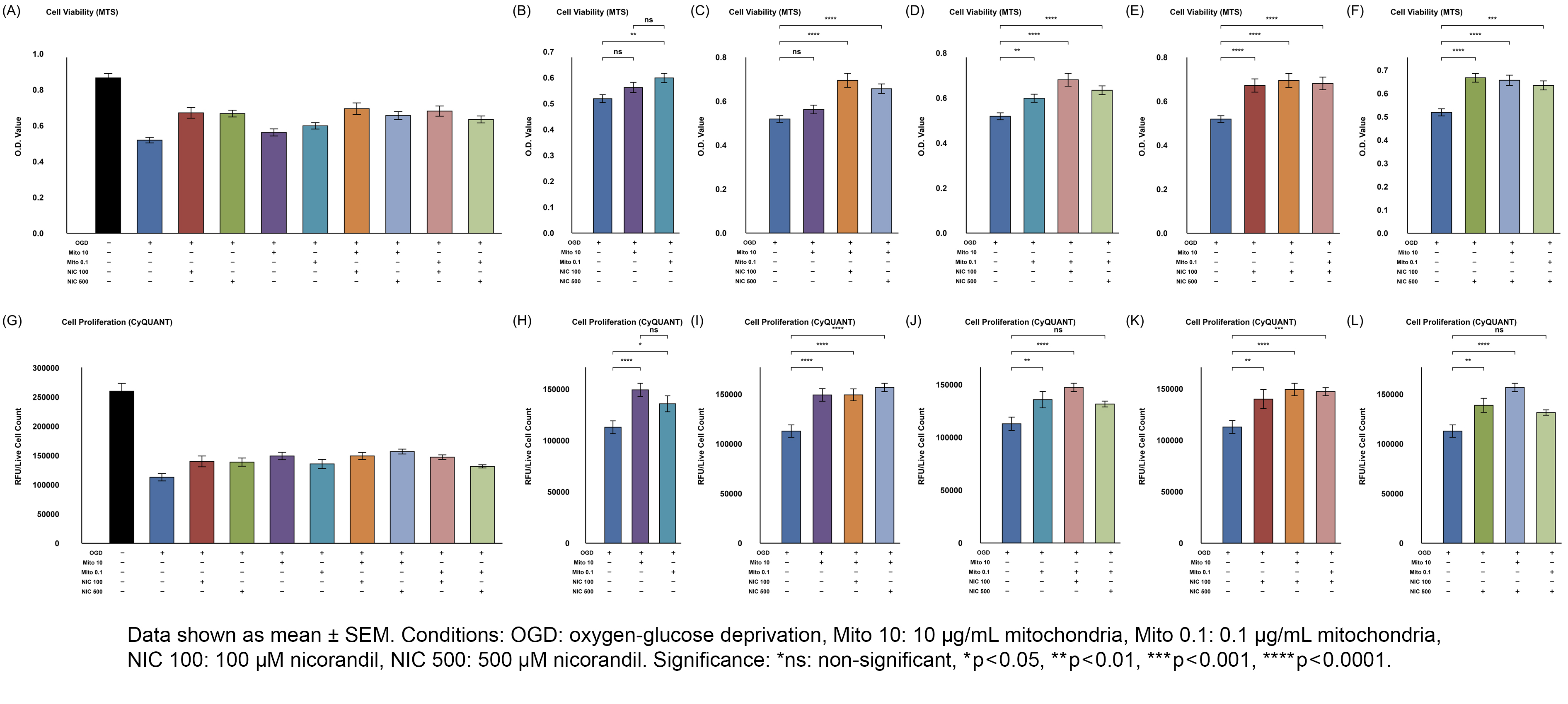
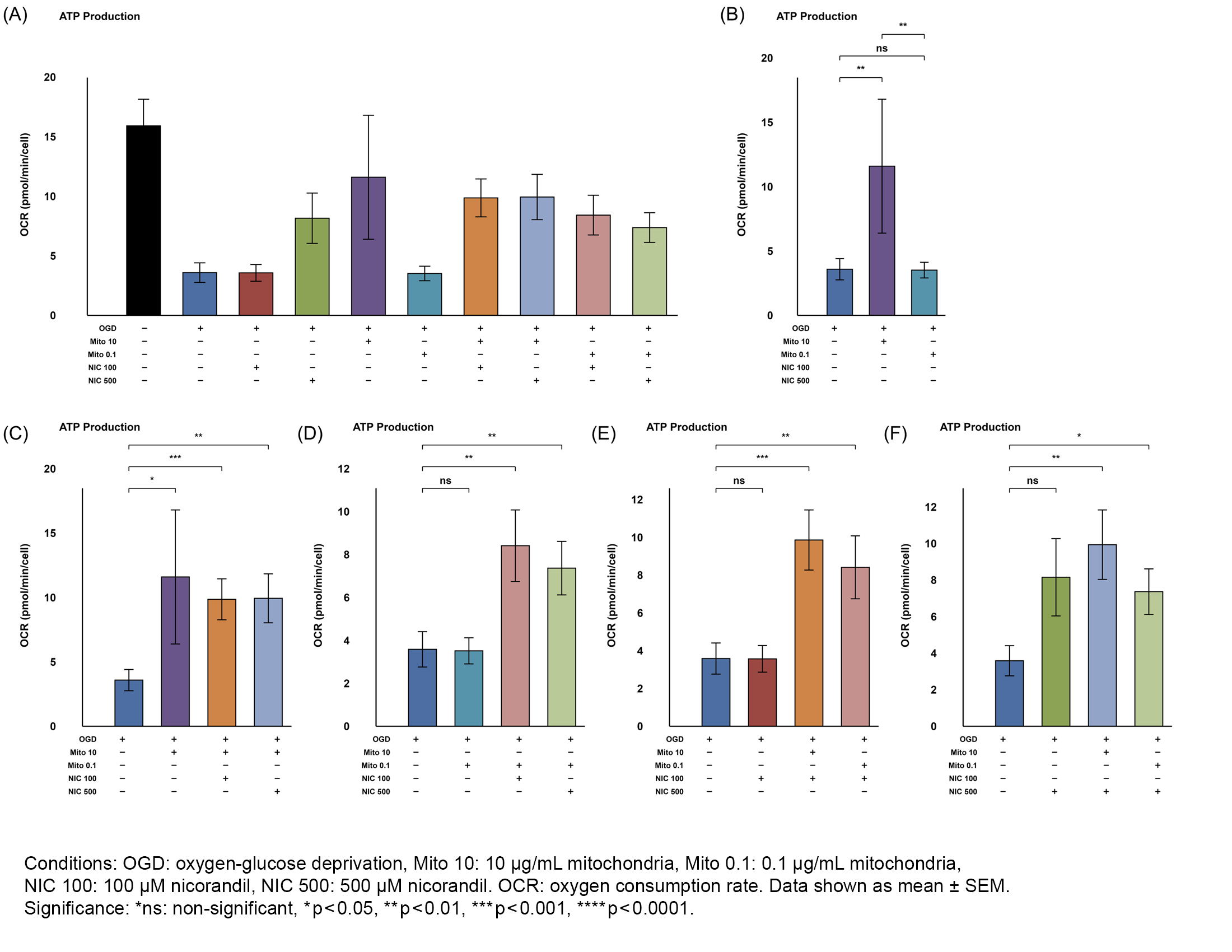
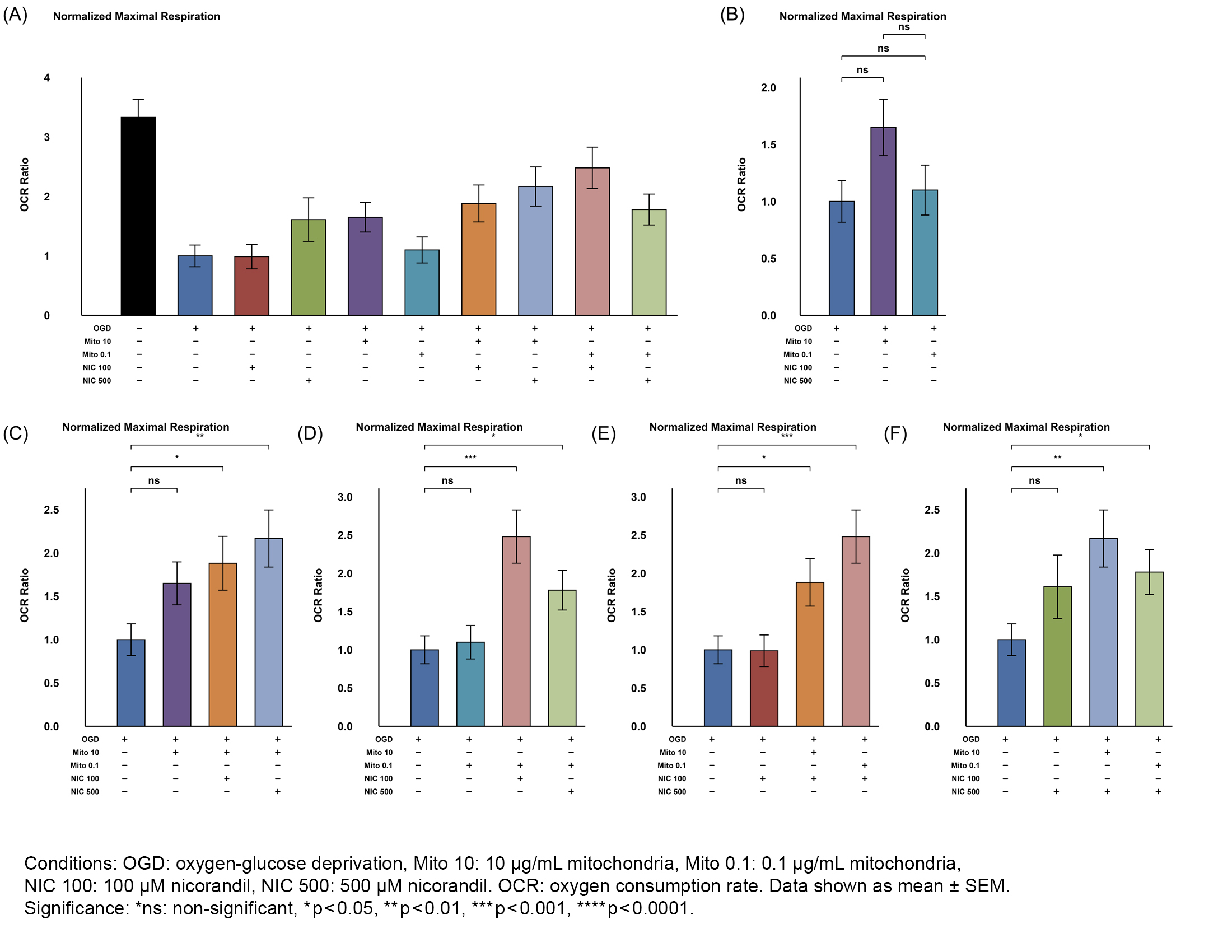
Results: Both Mito doses improved cell viability and proliferation in OGD-injured cells; 0.1µg/mL Mito significantly increased viability (MTS, p=0.001), while 10µg/mL showed borderline viability preservation (MTS, p=0.084) but significantly increased proliferation (CyQUANT, p<0.001). 10µg/mL Mito significantly preserved ATP production (p=0.020) and marginally improved maximal respiration (p=0.057); 0.1µg/mL did not. Both Mito doses with NIC further enhanced ATP production and basal/maximal respiration. Co-treatment with Mito and NIC shifted metabolism toward glycolysis compared to Mito alone, with reduced OCR/ECAR (p=0.007 for 10µg/mL; p=0.042 for 0.1µg/mL).
Conclusions: Skeletal muscle-derived MTx effectively preserved mitochondrial function and viability of HT-22 cells when given during ongoing OGD, suggesting a potentially new therapeutic window prior to reperfusion. NIC co-treatment further improved metabolic tolerance, indicating a possible synergistic effect. Overall, MTx has neuroprotective potential for stroke in aortic surgery.
Hypothesis/Aims: This study aimed to evaluate whether MTx during ongoing ischemia preserves neuronal viability and mitochondrial function in an in vitro oxygen-glucose deprivation (OGD) model, and whether NIC co-treatment provides a synergistic effect.
Methods: The study included two main groups: normoxic control and OGD; each had 9 subgroups: no treatment, 10 or 0.1µg/mL mitochondria (Mito), 100 or 500µM NIC, and their combinations. HT-22 neuronal cells were subjected to OGD for 10h, with Mito and/or NIC added 4h after initiation. Controls remained under normoxia. After OGD, all subgroups returned to normoxia for 17h. Mitochondria were isolated from mouse skeletal muscle. Cell viability, proliferation, and mitochondrial function were analyzed using MTS, CyQUANT, and Seahorse assays.
Results: Both Mito doses improved cell viability and proliferation in OGD-injured cells; 0.1µg/mL Mito significantly increased viability (MTS, p=0.001), while 10µg/mL showed borderline viability preservation (MTS, p=0.084) but significantly increased proliferation (CyQUANT, p<0.001). 10µg/mL Mito significantly preserved ATP production (p=0.020) and marginally improved maximal respiration (p=0.057); 0.1µg/mL did not. Both Mito doses with NIC further enhanced ATP production and basal/maximal respiration. Co-treatment with Mito and NIC shifted metabolism toward glycolysis compared to Mito alone, with reduced OCR/ECAR (p=0.007 for 10µg/mL; p=0.042 for 0.1µg/mL).
Conclusions: Skeletal muscle-derived MTx effectively preserved mitochondrial function and viability of HT-22 cells when given during ongoing OGD, suggesting a potentially new therapeutic window prior to reperfusion. NIC co-treatment further improved metabolic tolerance, indicating a possible synergistic effect. Overall, MTx has neuroprotective potential for stroke in aortic surgery.
More abstracts on this topic:
Attenuating Post-stroke Ischemia Reperfusion Injury: Establishing the Efficacy of Disodium Malonate in a Clinically Relevant Sheep Model
Sorby-adams Annabel, Murphy Mike, Sharkey Jessica, Prag Hiran, Turner Renee, Skein Keziah, Guglietti Bianca, Pullan Caitlin, Williams Georgia, Krieg Thomas
Anatomic Physiological Scoring is a Comparable Predictor of Adult Congenital Operative Morbidity and MortalityLa Brenda, Taylor-fishwick Jon, Macbeth Morgan, Soohoo Megan