Final ID: Su4039
X-Chromosome-Wide Association Study Identifies Novel Heart Failure Risk Loci with Sex- and Subtype-Specific Effects
Abstract Body (Do not enter title and authors here): Background/Aims: Heart failure (HF) is a leading cause of cardiovascular morbidity and mortality, with notable sex differences. Although the lifetime risk of HF is similar between sexes, HF with preserved ejection fraction (HFpEF) is more common in women, while HF with reduced ejection fraction (HFrEF) predominantly affects men. While genome-wide association studies have identified over 50 loci associated with HF and its subtypes, previous work has focused on autosomes, leaving the X chromosome underexplored despite its biological relevance and clear sex differences in HF. This study presents the first sex-stratified X-chromosome-wide association study (XWAS) of unclassified HF, HFrEF and HFpEF in a multi-ancestry cohort from the Million Veteran Program (MVP).
Methods: We analyzed X-chromosome genotype data from 658,582 MVP participants (91.6% male) across four ancestries: European (72.1%), African American (19.5%), Hispanic (7.5%), and Asian (0.9%). Genotyping was performed using Axiom Biobank Array. Sex- and ancestry- stratified XWAS was conducted using XWAS 3.0 using logistical regression models adjusting for age and the top 10 principal components. Multi-ancestry results were meta-analyzed separately for male and females using GWAMA, incorporating a random-effects model to account for heterogeneity across ancestries. Statistical significance was defined using Bonferroni correction.
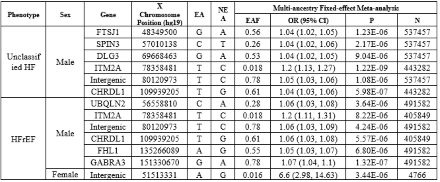
Results: In unclassified HF, a total of 6 loci were identified in males. For HFrEF, 6 loci were identified in males and 1 in females, while no significant loci were identified for HFpEF. (Table 1). Notably, the genomic loci harboring CHRDL1 and ITM2A were significantly associated with both unclassified HF and HFrEF, but only in males. One intergenic locus identified in females with HFrEF indicates a possible female-specific genetic contribution to HF risk. These findings reflect sex-specific genetic pathways potentially involved in cardiac remodeling and immune regulation in HF and its major sub-types. The smaller number of loci identified in females compared to males may be attributable to the predominantly male composition of the MVP cohort.
Conclusions: These results emphasize the need to include the X chromosome in HF genetic studies. The discovery of variants with sex- and subtype-specific effects underscores the role of X-linked genes in HF pathophysiology and supports precision medicine approaches tailored by sex and HF subtype.
Methods: We analyzed X-chromosome genotype data from 658,582 MVP participants (91.6% male) across four ancestries: European (72.1%), African American (19.5%), Hispanic (7.5%), and Asian (0.9%). Genotyping was performed using Axiom Biobank Array. Sex- and ancestry- stratified XWAS was conducted using XWAS 3.0 using logistical regression models adjusting for age and the top 10 principal components. Multi-ancestry results were meta-analyzed separately for male and females using GWAMA, incorporating a random-effects model to account for heterogeneity across ancestries. Statistical significance was defined using Bonferroni correction.
Results: In unclassified HF, a total of 6 loci were identified in males. For HFrEF, 6 loci were identified in males and 1 in females, while no significant loci were identified for HFpEF. (Table 1). Notably, the genomic loci harboring CHRDL1 and ITM2A were significantly associated with both unclassified HF and HFrEF, but only in males. One intergenic locus identified in females with HFrEF indicates a possible female-specific genetic contribution to HF risk. These findings reflect sex-specific genetic pathways potentially involved in cardiac remodeling and immune regulation in HF and its major sub-types. The smaller number of loci identified in females compared to males may be attributable to the predominantly male composition of the MVP cohort.
Conclusions: These results emphasize the need to include the X chromosome in HF genetic studies. The discovery of variants with sex- and subtype-specific effects underscores the role of X-linked genes in HF pathophysiology and supports precision medicine approaches tailored by sex and HF subtype.
More abstracts on this topic:
Acute Administration of The Novel Cardiac Sarcomere Modulator EDG-7500, Improves Ventricular Filling While Preserving LVEF In Dogs with Pacing Induced Left-Ventricular Systolic Dysfunction
Evanchik Marc, Emter Craig, Del Rio Carlos, Roof Steve, St Clair Sydney, Russell Alan, Henze Marcus, Semigran Marc
A Case of Dilated Cardiomyopathy and Systemic Thromboembolism in a Young Patient on Testosterone Replacement TherapySabri Muhammad, Ijaz Naila, Nadeem Ramsha, Checchio Lucy, Riaz Faiza