Final ID: 4367120
Impact of Aortic Valve Calcification on Functional Valve Area and Cardiac Structure and Function in a Phase 2 Trial of Ataciguat
Abstract Body (Do not enter title and authors here): Background:
Calcific aortic valve stenosis (CAVS) is characterized by the progressive accrual of aortic valvular calcium (AVC), resulting in impaired valvular compliance and mechanics and impaired cardiac function leading to heart failure (HF). In a phase 2 trial of 23 patients with moderate CAVS, ataciguat (ATA), a soluble guanylate cyclase activator, relative to placebo slowed the deposition of AVC, decreases in aortic valve area (AVA; by continuity equation), and increases in diastolic dysfunction and left ventricular (LV) mass. ATA may have other favorable effects on ventricular, valvular, and/or vascular pathophysiology relevant to patients with CAVS.
Hypothesis:
We hypothesized that slowing the rate of AVC deposition is associated with improvements in valvular compliance and in measures of cardiac structure and function.
Methods:
A phase 2 study randomized patients with moderate CAVS 1:1 to receive ATA 200 mg/day or placebo for up to 12 months (NCT02481258). The primary endpoint was change in AVC assessed by cardiac CT, and measures of aortic valve function and cardiac structure and function were also assessed. AVA was calculated by the Modified Gorlin (Hakki) equation to additionally assess valvular compliance. Correlations between AVC, AVA, and other measures of cardiac structure and function were assessed by exploratory linear mixed models.
Results:
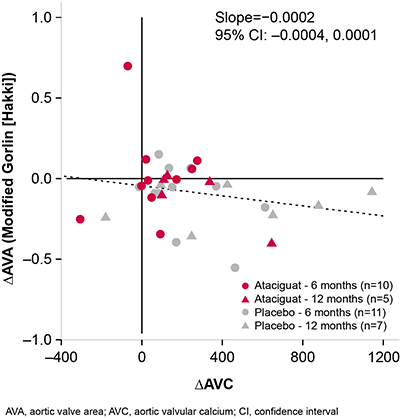
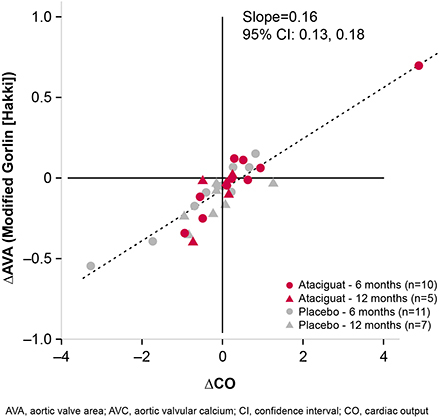
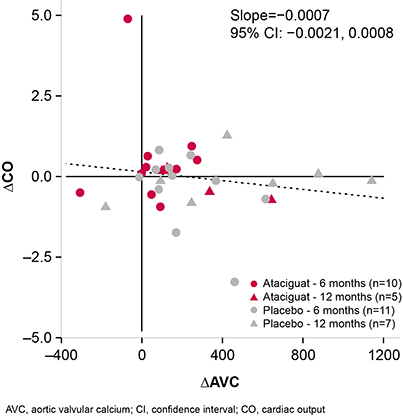
Changes in AVC from baseline were negatively correlated with changes in AVA (slope = −0.0002 [95% CI −0.0004, 0.0001]; Figure 1), such that patients with the least AVC deposition had minimal changes in AVA. Reciprocally, changes from baseline in AVA were positively correlated with changes in cardiac output (CO; slope = 0.16 [95% CI 0.13, 0.18]; Figure 2). Treatment with ATA, compared with placebo, was associated with improvements in systolic function. Changes in AVC deposition correlated with changes in CO (slope = −0.0007 [95% CI −0.0021, 0.0008]; Figure 3), and measured improvements in CO were generally more observed in patients with less of an increase in AVC, which were more frequently observed in those treated with ATA compared with placebo.
Conclusions:
These data suggest that slowing the rate of AVC deposition with ATA may result in improvements in CO through improved myocardial function and valvular compliance. Larger controlled trials are needed to assess such favorable myocardial/valvular effects that may help to preserve functional capacity and slow progression to HF.
Calcific aortic valve stenosis (CAVS) is characterized by the progressive accrual of aortic valvular calcium (AVC), resulting in impaired valvular compliance and mechanics and impaired cardiac function leading to heart failure (HF). In a phase 2 trial of 23 patients with moderate CAVS, ataciguat (ATA), a soluble guanylate cyclase activator, relative to placebo slowed the deposition of AVC, decreases in aortic valve area (AVA; by continuity equation), and increases in diastolic dysfunction and left ventricular (LV) mass. ATA may have other favorable effects on ventricular, valvular, and/or vascular pathophysiology relevant to patients with CAVS.
Hypothesis:
We hypothesized that slowing the rate of AVC deposition is associated with improvements in valvular compliance and in measures of cardiac structure and function.
Methods:
A phase 2 study randomized patients with moderate CAVS 1:1 to receive ATA 200 mg/day or placebo for up to 12 months (NCT02481258). The primary endpoint was change in AVC assessed by cardiac CT, and measures of aortic valve function and cardiac structure and function were also assessed. AVA was calculated by the Modified Gorlin (Hakki) equation to additionally assess valvular compliance. Correlations between AVC, AVA, and other measures of cardiac structure and function were assessed by exploratory linear mixed models.
Results:
Changes in AVC from baseline were negatively correlated with changes in AVA (slope = −0.0002 [95% CI −0.0004, 0.0001]; Figure 1), such that patients with the least AVC deposition had minimal changes in AVA. Reciprocally, changes from baseline in AVA were positively correlated with changes in cardiac output (CO; slope = 0.16 [95% CI 0.13, 0.18]; Figure 2). Treatment with ATA, compared with placebo, was associated with improvements in systolic function. Changes in AVC deposition correlated with changes in CO (slope = −0.0007 [95% CI −0.0021, 0.0008]; Figure 3), and measured improvements in CO were generally more observed in patients with less of an increase in AVC, which were more frequently observed in those treated with ATA compared with placebo.
Conclusions:
These data suggest that slowing the rate of AVC deposition with ATA may result in improvements in CO through improved myocardial function and valvular compliance. Larger controlled trials are needed to assess such favorable myocardial/valvular effects that may help to preserve functional capacity and slow progression to HF.
More abstracts on this topic:
Aortic Root Pressure for Detecting Aortic Stenosis using Machine Learning
Dunn Michael, Lalush David, Wheaten Sterling, Stouffer George, Syed Faisal
Aortic Root Pressure for Detecting Aortic Stenosis using Machine LearningDunn Michael, Lalush David, Wheaten Sterling, Stouffer George, Syed Faisal