Final ID: MP1853
Plasmalemma Vesicle-Associated Protein (PLVAP) Maintains Cardiac Endothelial Barrier function via ARNT-dependent mechanism
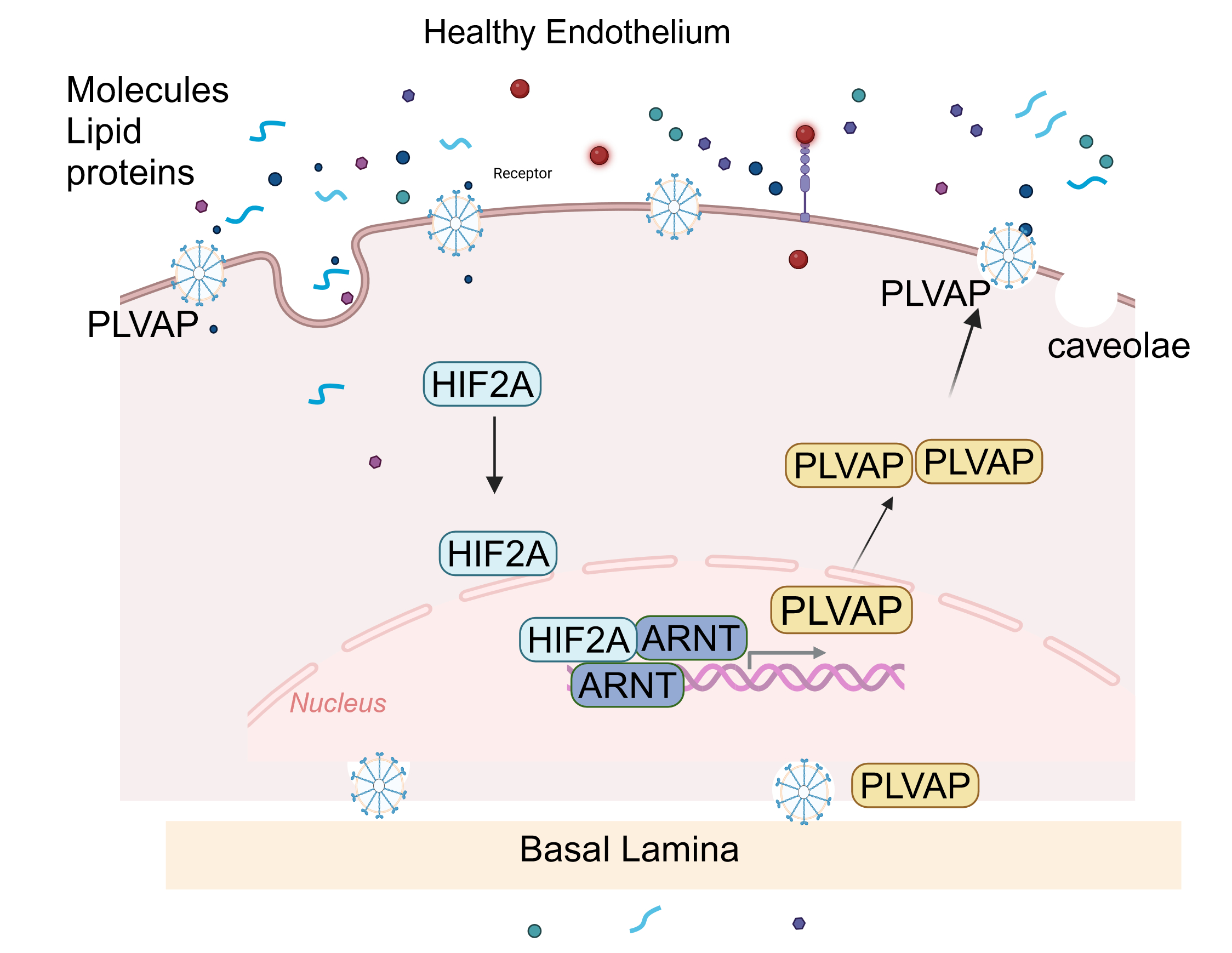
Abstract Body (Do not enter title and authors here): Background Microvascular endothelial dysfunction is a key contributor to cardiac injury in cardiovascular diseases. Plasmalemma vesicle-associated protein (PLVAP) is a structural component of endothelial diaphragms and is essential for vascular development. In murine models, disruption of the PLVAP gene leads to embryonic lethality, marked by edema, hemorrhage, and significant structural defects in subcutaneous capillaries; however, its upstream regulation and role in cardiac endothelial barrier function remain poorly understood. HIF2A/ARNT heterodimers are essential regulators of endothelial cell (EC) behavior, affecting their barrier function. Given the central role of endothelial dysfunction in various diseases, defining the ARNT-PLVAP pathway may reveal new therapeutic targets for cardiac and vascular complications
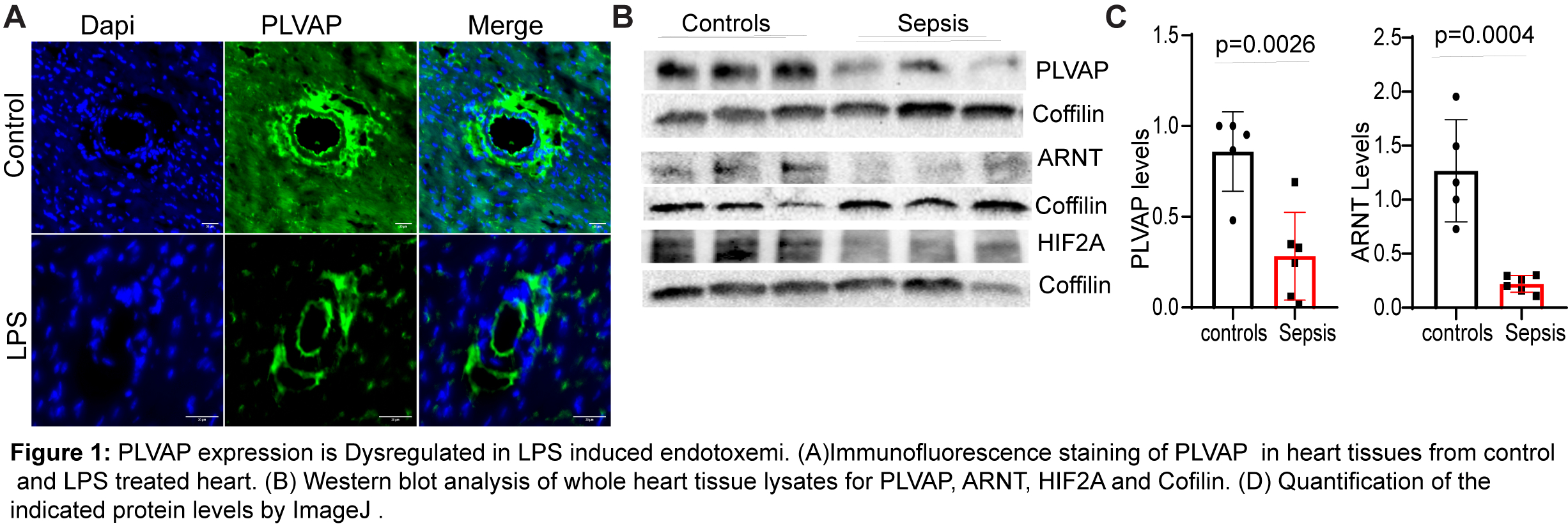
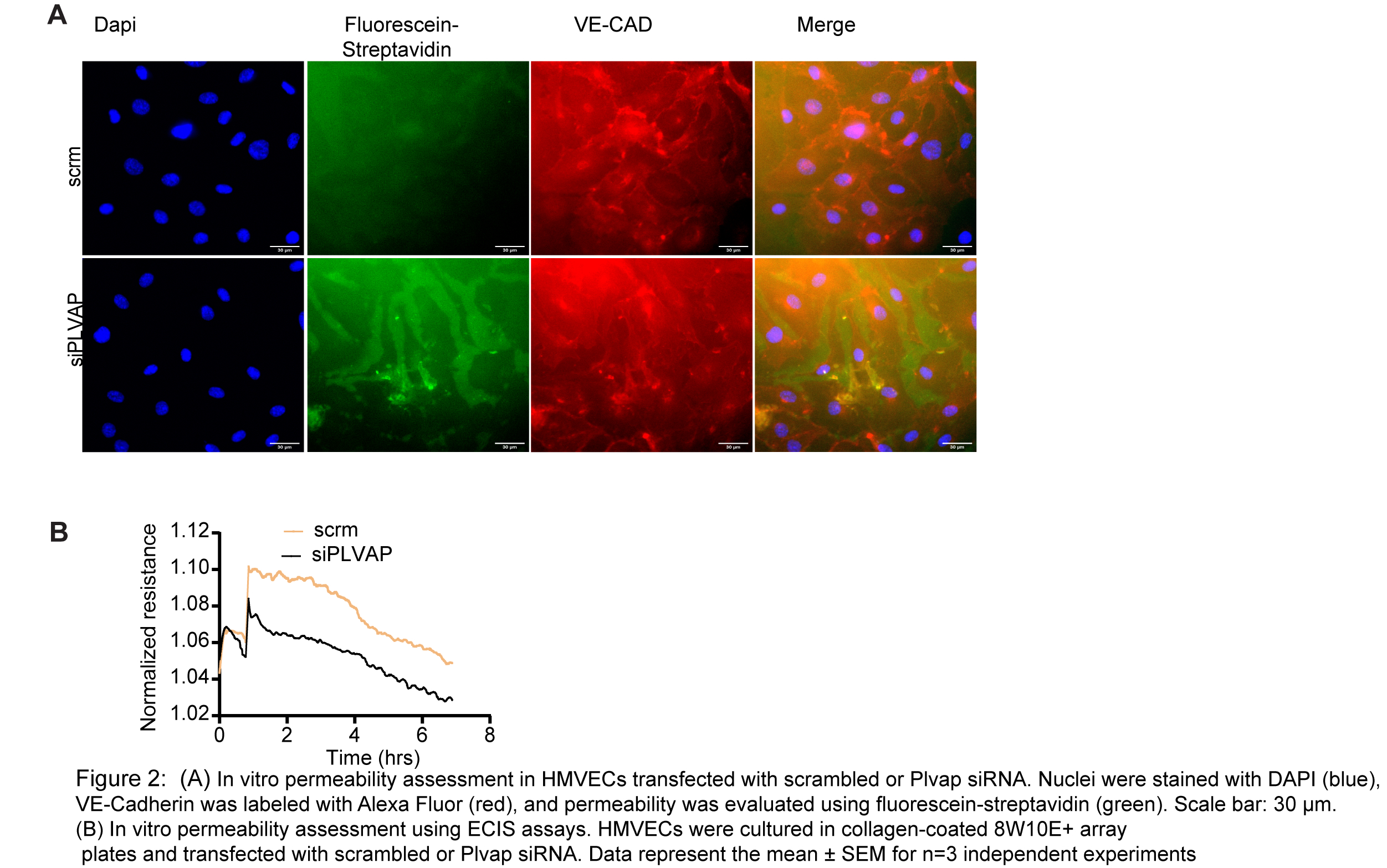
Methods and Results We used tamoxifen-inducible, endothelial-specific knockout mice lacking either HIF-2α or ARNT (Hif2αflox/flox or ARNTflox/flox driven by Cdh5-CreERT2). Primary cardiac microvascular endothelial cells (CMVECs) were isolated from these mice, and human CMVECs were transfected with siRNA targeting HIF2A, ARNT, or PLVAP to assess gene regulation and endothelial barrier function. Loss of either HIF2A or ARNT significantly reduced PLVAP expression at both mRNA and protein levels (n = 6, p < 0.001) and increased cardiac vascular leakage in an LPS-induced endotoxemia model compared to wild-type controls (LPS 10mg/kg body weight) (n = 10–12, p < 0.05). Mechanistic studies using ChIP, Co-IP, and luciferase assays demonstrated that ARNT directly regulates PLVAP transcription via promoter binding. PLVAP deletion impaired endothelial glycocalyx and reduced VE-cadherin and Occludin expression, suggesting its essential role in maintaining vascular barrier function. Furthermore, LPS treatment (500ng/ml) in human CMVECs suppressed PLVAP expression, while lentivirus-mediated overexpression of ARNT restored PLVAP expression and rescued endothelial resistance, as measured by ECIS, confirming ARNT’s protective role in inflammatory injury.
Conclusion: PLVAP is a key transcriptional target of the HIF-2α/ARNT pathway and is essential for maintaining cardiac endothelial barrier integrity, especially under inflammatory conditions. Disruption of this axis leads to vascular leakage, while its restoration reverses barrier dysfunction, highlighting the ARNT-PLVAP pathway as a potential therapeutic target in cardiovascular disease.
Methods and Results We used tamoxifen-inducible, endothelial-specific knockout mice lacking either HIF-2α or ARNT (Hif2αflox/flox or ARNTflox/flox driven by Cdh5-CreERT2). Primary cardiac microvascular endothelial cells (CMVECs) were isolated from these mice, and human CMVECs were transfected with siRNA targeting HIF2A, ARNT, or PLVAP to assess gene regulation and endothelial barrier function. Loss of either HIF2A or ARNT significantly reduced PLVAP expression at both mRNA and protein levels (n = 6, p < 0.001) and increased cardiac vascular leakage in an LPS-induced endotoxemia model compared to wild-type controls (LPS 10mg/kg body weight) (n = 10–12, p < 0.05). Mechanistic studies using ChIP, Co-IP, and luciferase assays demonstrated that ARNT directly regulates PLVAP transcription via promoter binding. PLVAP deletion impaired endothelial glycocalyx and reduced VE-cadherin and Occludin expression, suggesting its essential role in maintaining vascular barrier function. Furthermore, LPS treatment (500ng/ml) in human CMVECs suppressed PLVAP expression, while lentivirus-mediated overexpression of ARNT restored PLVAP expression and rescued endothelial resistance, as measured by ECIS, confirming ARNT’s protective role in inflammatory injury.
Conclusion: PLVAP is a key transcriptional target of the HIF-2α/ARNT pathway and is essential for maintaining cardiac endothelial barrier integrity, especially under inflammatory conditions. Disruption of this axis leads to vascular leakage, while its restoration reverses barrier dysfunction, highlighting the ARNT-PLVAP pathway as a potential therapeutic target in cardiovascular disease.
More abstracts on this topic:
ACLY Inhibition as a Novel Therapeutic Approach for Vascular Remodeling in Coronary Artery Disease.
Grobs Yann, Reem El-kabbout, Potus Francois, Provencher Steeve, Boucherat Olivier, Bonnet Sebastien, Romanet Charlotte, Lemay Sarah-eve, Bourgeois Alice, Voisine Pierre, Theberge Charlie, Sauvaget Melanie, Breuils Bonnet Sandra, Martineau Sandra
A Case of Steroid-Refractory Immune-checkpoint-inhibitor Induced Myocarditis Responsive to Mycophenolate and Anti-thymocyte globulinDabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey