Final ID: MP1869
Selnoflast reduces markers of systemic inflammation in participants with coronary artery disease and elevated C-reactive protein
While low-density lipoprotein (LDL) cholesterol lowering is central to coronary artery disease (CAD) management, elevated interleukin-6 (IL-6) and high-sensitivity CRP (hsCRP) in statin-treated patients signals residual NLRP3-driven inflammation and ongoing risk.
Hypothesis
We hypothesized that selnoflast, an oral NLRP3 inflammasome inhibitor, is safe and would significantly reduce IL-6 and hsCRP levels in participants with CAD and elevated hsCRP.
Methods
This randomized, double-blinded, placebo-controlled trial (ISRCTN10520571) assessed the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of selnoflast (200 mg po BID) in 22 participants, with history of myocardial infarction and elevated hsCRP (≥2 mg/L), randomized 1:1 to receive selnoflast or placebo (PBO). The primary endpoint was safety; secondary and exploratory endpoints included PK and PD assessment of IL-6 and hsCRP change from baseline (BL).
Results
Among the 22 participants, mean (standard deviation [SD]) age was 64.5 (7.6) years, 11 (50%) were female, and BL mean (SD) IL-6 and hsCRP were 1.79 (28.24) pg/mL and 6.36 (4.52) mg/L, respectively.
Selnoflast demonstrated a favorable safety profile, consistent with prior studies. Seventeen adverse events (AEs) were reported: 11 AEs in 3 of 11 (27.3%) participants on selnoflast and 6 AEs in 5 of 11 (45.5%) participants on PBO. On Day 15, mean (SD) plasma concentrations of selnoflast were 5.31 (3.68) μg/mL pre-dose and 10.5 (4.62) μg/mL at 2 hours post-dose (Cmax). Steady-state PK was approached by Day 2, with <2-fold accumulation of mean Cmax from Day 1 6.31 (3.68) μg/mL to Day 15 10.5 (4.62) μg/mL.
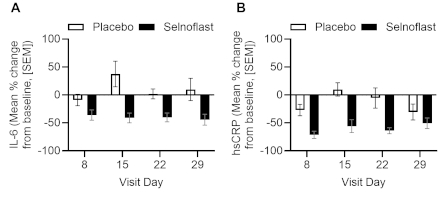
IL-6 levels were comparable at BL, with greater mean reductions by Day 8 in the selnoflast group (–36% change, 13.27±31.22 pg/mL) vs PBO (–9.03% change, 6.57±5.85 pg/mL). Absolute reductions remained ≥36% through Day 29, with individual PBO-corrected IL-6 decreases ranging from 27-79% (Fig 1A). hsCRP levels were also comparable at BL, with greater mean reductions by Day 8 in the selnoflast group (–70.95% change, 1.35±1.01 mg/L) vs PBO (–29.45% change, 4.99±5.59 mg/L). Absolute reductions remained ≥55% through Day 29, with individual PBO-corrected decreases ranging from 25-66% (Fig 1B).
Conclusion
Selnoflast was well tolerated without notable safety signals relative to PBO, approached steady-state PK by Day 2, and demonstrated consistent reductions in inflammatory biomarkers IL-6 and hsCRP in patients with CAD and residual inflammation.
- Place, David ( Genentech , South San Francisco , California , United States )
- Paruchuri, Kaavya ( Mass General , Boston , Massachusetts , United States )
- Kunder, Rebecca ( Genentech , South San Francisco , California , United States )
- Israel-hanniford, Davelene ( Genentech , South San Francisco , California , United States )
- Li, Shawn ( Genentech , South San Francisco , California , United States )
- Garvin, Rachel ( Genentech , South San Francisco , California , United States )
- Natarajan, Pradeep ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Libby, Peter ( BRIGHAM AND WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Li, Yihao ( Genentech , South San Francisco , California , United States )
- Zhang, Lu ( Genentech , South San Francisco , California , United States )
- Tang, Fei ( Genentech , South San Francisco , California , United States )
- Nguyen, Allen ( Genentech , South San Francisco , California , United States )
- Shim, Jeongsup ( Genentech , South San Francisco , California , United States )
- Banerjee, Prajna ( Genentech , South San Francisco , California , United States )
- Fong, Alice ( Genentech , South San Francisco , California , United States )
- Chu, Tom ( Genentech , South San Francisco , California , United States )
Meeting Info:
Session Info:
Vascular Inflammation and Resolution
Sunday, 11/09/2025 , 09:15AM - 10:25AM
Moderated Digital Poster Session
More abstracts on this topic:
Wilsgaard Tom, Rosamond Wayne, Schirmer Henrik, Lindekleiv Haakon, Attia Zachi, Lopez-jimenez Francisco, Leon David, Iakunchykova Olena
Additive Value of Lipoprotein(a), Remnant Cholesterol, and Inflammation for Risk Stratification of Myocardial Infarction: Evidence from the UK BiobankKazibwe Richard, Schaich Christopher, Kingsley Jeffrey, Rikhi Rishi, Namutebi Juliana, Chevli Parag, Mirzai Saeid, Shapiro Michael
More abstracts from these authors:
Small Aeron, Vijayakumar Shilpa, Cuddy Sarah, Libby Peter, Natarajan Pradeep, Gaggin Hanna, Dorbala Sharmila, Honigberg Michael
Premature Menopause and Polygenic Risk on Incident Coronary Artery Disease among Postmenopausal WomenGanesh Shriienidhie, Hornsby Whitney, Paruchuri Kaavya, Ruan Yunfeng, Patel Aniruddh, Honigberg Michael, Natarajan Pradeep