Final ID: 4365681
Cardiac adaptation to endurance exercise training requires suppression of GDF15 via PGC-1α
Approaches: We utilize a genetic mouse model of cardiomyocyte PGC-1α deficiency along with somatic overexpression and knockdown of a PGC-1α related protein GDF15 using adeno-associated virus. We further utilize neonatal rat ventricular myocytes, human single nucleus RNA sequencing of patients with cardiomyopathies, and whole exome sequencing of human participants from the UK BioBank to address the relationship of PGC-1α with GDF15 and with cardiac dysfunction.
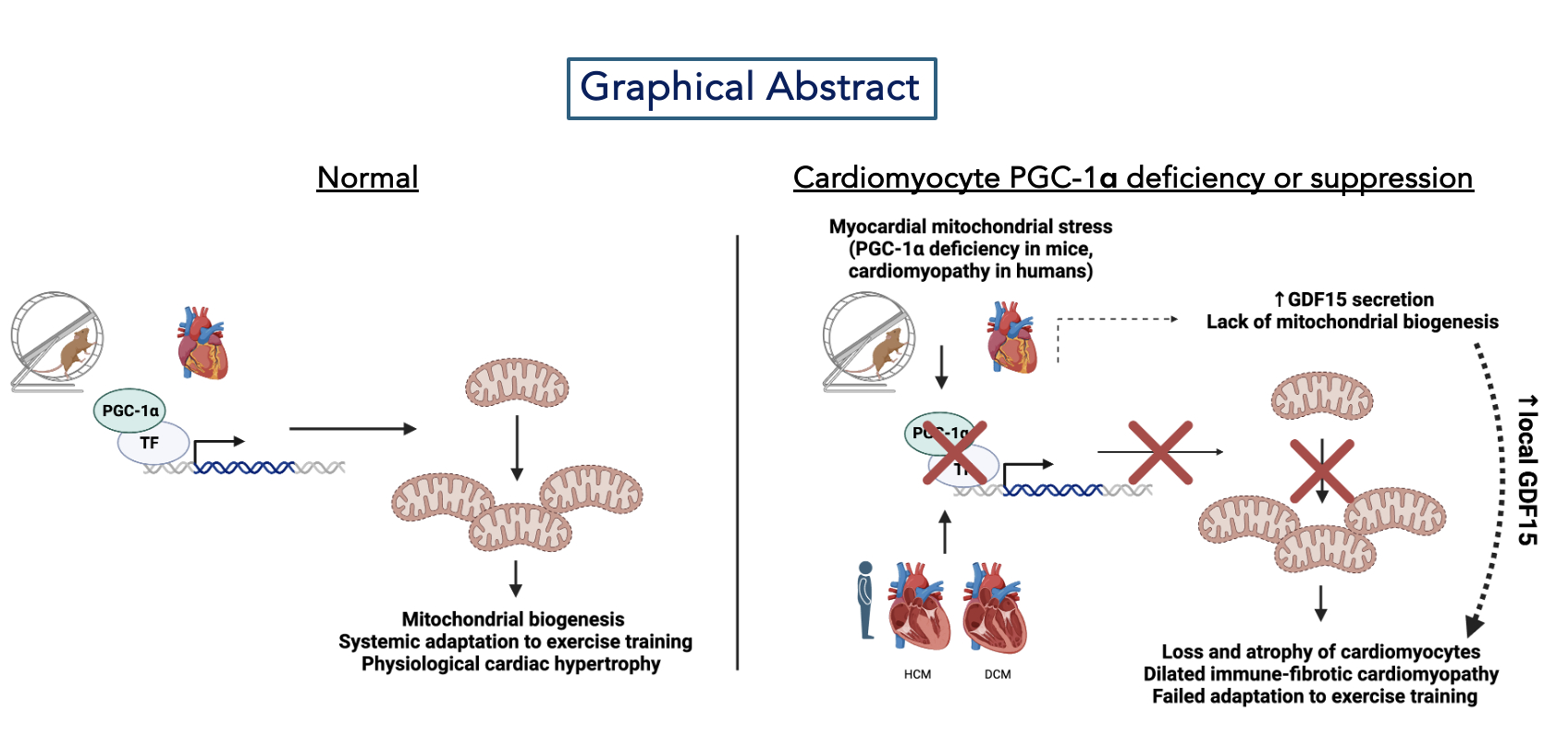
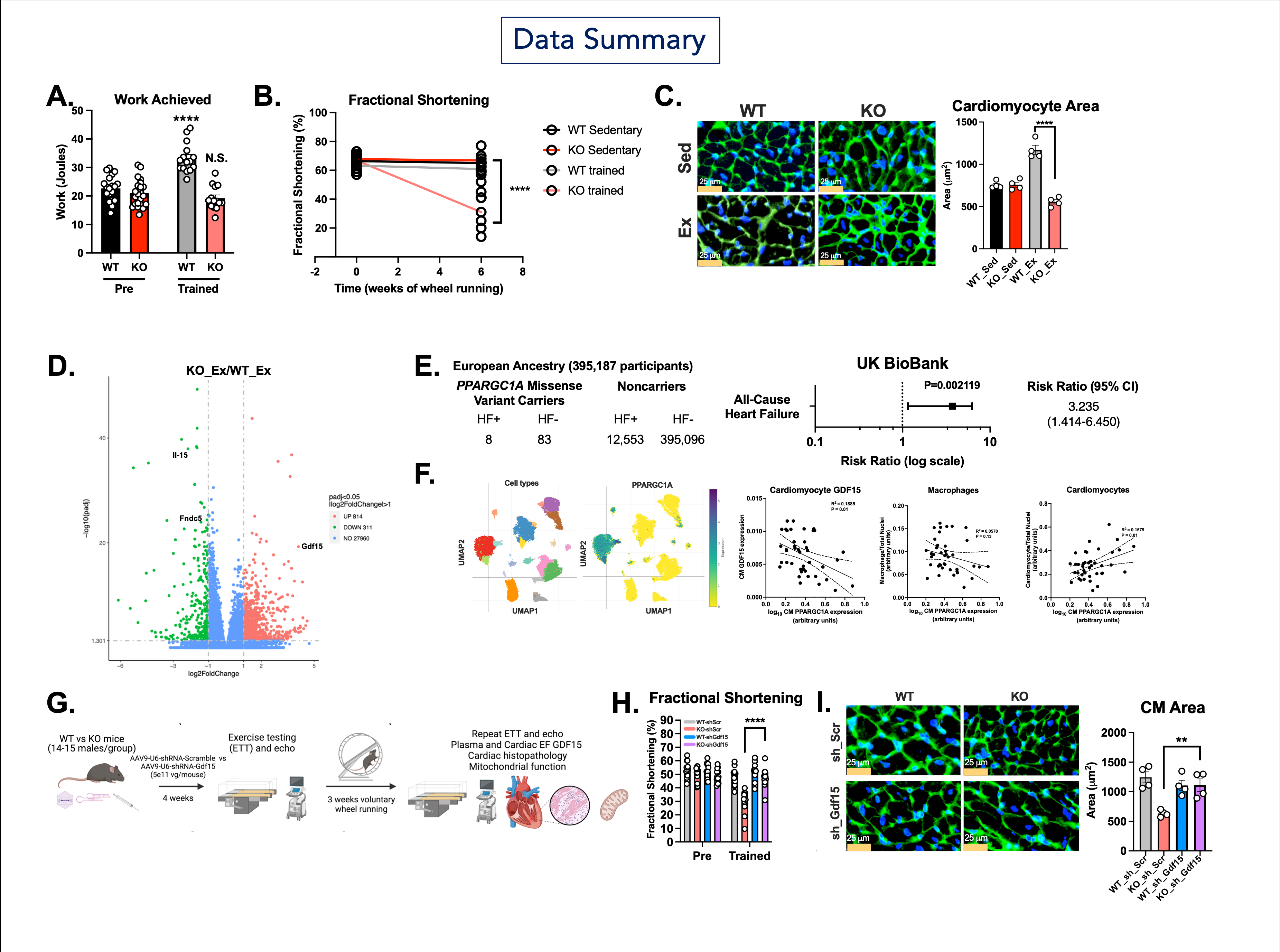
Results: Wild-type and cardiomyocyte PGC-1α KO mice were subjected to voluntary wheel running for 5 weeks. Mice ran comparably over that time. Despite this, cardiomyocyte PGC-1α KO mice demonstrated no improvement in peak exercise capacity compared to WT mice (exercise work 33 J in WT vs 19 J in KO, p<0.001). Instead, PGC-1α KO mice demonstrated resting dilated cardiomyopathy after just 5 weeks of training (cardiac fractional shortening after training 60% in WT vs. 31% in KO, p<0.0001). Supporting this, extremely rare protein human genetic coding variants in PPARGC1A are associated with all-cause heart failure in the UK BioBank (RR 3.23, 95% CI 1.41-6.45, p=0.002). Cardiomyocyte PGC-1α-deficient trained hearts demonstrated absence of physiological hypertrophy (area 1170 vs. 555 μm2, p<0.0001) and markedly increased expression of the myomitokine GDF15. GDF15 was secreted exclusively from cardiomyocytes but is not systemically elevated in PGC-1α-deficient mouse hearts. In cardiomyocytes, this occurs through the integrated stress response pathway, which is suppressed by PGC-1α overexpression. Cardiomyocyte-specific reduction of GDF15 preserves exercise tolerance, cardiac function, and exercise-induced cardiomyocyte hypertrophy in PGC-1α-deficient mice. We also find that cardiomyocyte PPARGC1A expression correlates with cardiomyocyte number and negatively with cardiomyocyte GDF15 expression in human cardiomyopathies through single nucleus RNA sequencing.
Conclusions: Our data implicate cardiomyocyte PGC-1α as a vital enabler of physiological adaptation to endurance exercise through suppression of GDF15-mediated cardiac dysfunction.
- Khetarpal, Sumeet ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Vargas Castillo, Ariana ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Smythers, Amanda ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Blackmore, Katherine ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Grauvogel, Louisa ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Mittenbuhler, Melanie ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Khandekar, Melin ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Curtin, Casie ( Beth Israel Deaconess , Boston , Massachusetts , United States )
- Wang, Chunyan ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Houstis, Nicholas ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Sprenger, Hans-georg ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Li, Haobo ( Massachusetts General Hospital , Malden , Massachusetts , United States )
- Jurgens, Sean ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Biddinger, Kiran ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Kuznetsov, Alexandra ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Freeman, Rebecca ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Ellinor, Patrick ( MGH- Cardiovascular Research Center , Boston , Massachusetts , United States )
- Nahrendorf, Matthias ( MGH- Cardiovascular Research Center , Boston , Massachusetts , United States )
- Paulo, Joao ( Harvard Medical School , Boston , Massachusetts , United States )
- Gygi, Steven ( Harvard Medical School , Boston , Massachusetts , United States )
- Dumesic, Phillip ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Asnani, Aarti ( Beth Israel Deaconess , Arlington , Massachusetts , United States )
- Vitale, Tevis ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Aragam, Krishna ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Puigserver, Pere ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Roh, Jason ( Massachusetts General Hospital , Brookline , Massachusetts , United States )
- Spiegelman, Bruce ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Rosenzweig, Anthony ( University of Michigan , Ann Arbor , Michigan , United States )
- Rhee, James ( MGH , Boston , Massachusetts , United States )
- Challa, Saketh ( MGH , Boston , Massachusetts , United States )
- Castro, Claire ( MGH- Cardiovascular Research Center , Boston , Massachusetts , United States )
- Pabel, Steffen ( MGH- Cardiovascular Research Center , Boston , Massachusetts , United States )
- Sun, Yizhi ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
- Bogoslavski, Dina ( Dana-Farber Cancer Institute , Boston , Massachusetts , United States )
Meeting Info:
Session Info:
Louis N. and Arnold M. Katz Basic Science Research Prize for Early Career Investigators Competition
Saturday, 11/08/2025 , 01:30PM - 02:45PM
Abstract Oral Session
More abstracts on this topic:
Helfer Abbigail, Sheehan Tara, Bursac Nenad
Heart Failure Exacerbates Renal Medullary Hypoxia and Increases Acute Kidney Injury Risk After Cardiopulmonary Bypass in Sheep.Trask-marino Anton, Lankadeva Yugeesh, Marino Bruno, Cochrane Andrew, Mccall Peter, Raman Jai, Furukawa Taku, Ow Connie, Booth Lindsea, May Clive
More abstracts from these authors:
Castro Claire, Lee Se-jin, Ellinor Patrick, Rosenzweig Anthony, Roh Jason, Bapat Aneesh, Hobson Ryan, Yu Andy, Li Haobo, Xiao Chunyang, Xia Peng, Yeri Ashish, Yu Paul
Placental-derived Senescence Associated Secretory Proteins Increase Atrial Fibrillation Susceptibility in PregnancyCastro Claire, Xiao Chunyang, Gray Kathryn, Edlow Andrea, Ellinor Patrick, Roh Jason