Final ID: MP913
Overcoming Barriers For Research Participation In Minority Patients In Lp(a)FRONTIERS EXPANSION: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study To Evaluate Efficacy And Safety Of Pelacarsen In U.S. Black And Hispanic Patients with Elevated Lp(a) And Established ASCVD
Abstract Body (Do not enter title and authors here): Background: Despite increased atherosclerotic cardiovascular disease (ASCVD) risk, Black and Hispanic individuals are underrepresented in ASCVD clinical trials. Black individuals have the highest prevalence and median levels of elevated lipoprotein(a) [Lp(a)] among U.S. populations, contributing to a significantly higher ASCVD risk.
Aim: Address and mitigate systemic barriers to clinical trial participation for historically underrepresented populations to enhance diversity and inclusion in cardiovascular research.
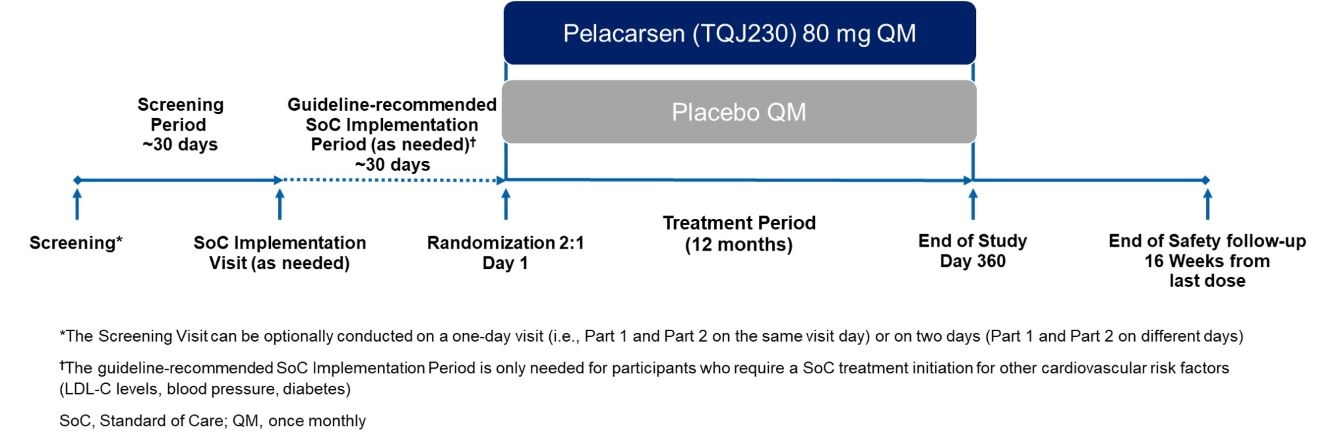
Methods: The Lp(a)FRONTIERS EXPANSION trial is a randomized, double-blind, phase 3b multicenter study evaluating the efficacy of pelacarsen, an Lp(a)-targeted therapy, vs placebo in lowering Lp(a) levels in U.S. Black and Hispanic patients with elevated Lp(a) (≥125 nmol/L) and established ASCVD. To increase trial participation in minority populations, the study used a 2:1 randomization ratio, increasing the chance of receiving the investigational treatment. Considering disparities in healthcare access and quality, the trial included a post-screening standard-of-care (SoC) visit, if needed, to optimize participants’ modifiable ASCVD risk factors. To reduce patient burden, required on-site visits were minimized. The trial aimed to enroll 400 participants across 150 strategically selected sites, including those in underserved areas, to improve accessibility and reduce travel time. Additional patient support included transportation assistance, childcare reimbursement and flexible scheduling. Sites were compensated for additional screening time, particularly to accommodate family involvement in decision-making, which is culturally significant in Hispanic communities.
Results: A total of 423 participants were enrolled over 11 months at 103 sites, a full year ahead of schedule. Of these, 65% identified as Black non-Hispanic, 30% as Hispanic and 5% as Black Hispanic. Women comprised 49% of the cohort; mean age was 63.2 years. Sixty participants received the optional SoC visit, with 37 initiating or adjusting treatment for ASCVD risk factors.
Conclusion: The Lp(a)FRONTIERS EXPANSION trial successfully demonstrated that deliberate, culturally tailored strategies can improve participation of minority populations, underrepresented in cardiovascular clinical trials. These results highlight the importance of inclusive trial design and operational efforts to achieve equitable representation in clinical research, ensuring generalizability of trial results.
Aim: Address and mitigate systemic barriers to clinical trial participation for historically underrepresented populations to enhance diversity and inclusion in cardiovascular research.
Methods: The Lp(a)FRONTIERS EXPANSION trial is a randomized, double-blind, phase 3b multicenter study evaluating the efficacy of pelacarsen, an Lp(a)-targeted therapy, vs placebo in lowering Lp(a) levels in U.S. Black and Hispanic patients with elevated Lp(a) (≥125 nmol/L) and established ASCVD. To increase trial participation in minority populations, the study used a 2:1 randomization ratio, increasing the chance of receiving the investigational treatment. Considering disparities in healthcare access and quality, the trial included a post-screening standard-of-care (SoC) visit, if needed, to optimize participants’ modifiable ASCVD risk factors. To reduce patient burden, required on-site visits were minimized. The trial aimed to enroll 400 participants across 150 strategically selected sites, including those in underserved areas, to improve accessibility and reduce travel time. Additional patient support included transportation assistance, childcare reimbursement and flexible scheduling. Sites were compensated for additional screening time, particularly to accommodate family involvement in decision-making, which is culturally significant in Hispanic communities.
Results: A total of 423 participants were enrolled over 11 months at 103 sites, a full year ahead of schedule. Of these, 65% identified as Black non-Hispanic, 30% as Hispanic and 5% as Black Hispanic. Women comprised 49% of the cohort; mean age was 63.2 years. Sixty participants received the optional SoC visit, with 37 initiating or adjusting treatment for ASCVD risk factors.
Conclusion: The Lp(a)FRONTIERS EXPANSION trial successfully demonstrated that deliberate, culturally tailored strategies can improve participation of minority populations, underrepresented in cardiovascular clinical trials. These results highlight the importance of inclusive trial design and operational efforts to achieve equitable representation in clinical research, ensuring generalizability of trial results.
More abstracts on this topic:
Assessing Racial Disparities in Heart Transplant Allocations Post-2018 Policy Change
Malkani Kabir, Zhang Ruina, Li Han, Ezema Ashley, Steitieh Diala, Purkayastha Subhanik, Kini Vinay
Impact of Lipoprotein(a) on Aortic Valve Replacement in Chinese Patients with Mild-to-Moderate Calcific Aortic Valve StenosisLu Mengying, Wang Xiao, Dou Kefei, Wu Naqiong, Li Zhi Fan, Yin Zheng, Li Xi, Zhang Wenjia, Luo Fang, Liu Xiaoning, Xu Yanlu, Liu Chen