Final ID: MP1110
Time-Course Single-Cell RNA Sequencing of Patient iPSC-Derived Heart Tissue Reveals Disease-Driving Pathways in Duchenne Muscular Dystrophy–Related Cardiomyopathy
Abstract Body (Do not enter title and authors here): Background and Aims: Duchenne muscular dystrophy (DMD) leads to fatal cardiomyopathy caused by mutations in the dystrophin gene. Gene therapy for the heart is limited due to poor delivery efficiency. In addition, patients are ineligible for heart transplants due to scoliosis and respiratory complications. Given the lack of effective therapies, efforts to clarify the mechanisms of DMD cardiomyopathy have been hampered by the limited availability of cardiac specimens. Here, we aimed to identify key driver pathways for DMD cardiomyopathy by constructing multicellular engineered heart tissue (EHT) under disease-relevant stress.
Methods: EHTs were generated from patient iPSC-derived cardiomyocytes and epicardial cell–derived non-cardiomyocytes. An isogenic control with a corrected dystrophin mutation was used as comparison. To induce an adult-like disease phenotype, we shifted cardiomyocyte metabolism toward fatty acid oxidation and applied pathological stress. Single-cell RNA sequencing (scRNA-seq) was conducted before and after pathological stimulation to capture disease-associated molecular changes.
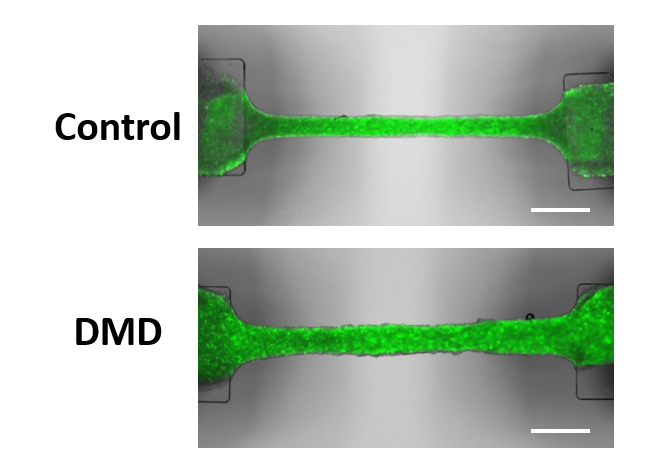
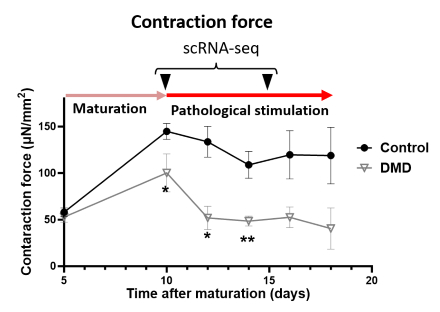
Results: Phenotypic assessment revealed that while control EHTs showed increased contractility during maturation, DMD EHTs lacked this improvement (p < 0.05) and exhibited disorganized sarcomeres (p < 0.01). Upon pathological stimulation with mechanical stress and inflammatory cytokines, DMD EHTs showed a further decline in contractility (p < 0.01), accompanied by increased fibrosis (p < 0.05) and DNA damage. TFvelocity analysis of time-course scRNA-seq revealed that DMD cardiomyocytes exhibited a gene expression profile resembling that of dilated cardiomyopathy. This analysis identified early activation of pathways related to microtubule instability and fibrosis, followed by later activation of pathways associated with responses to oxidative stress and inflammation, as well as muscle atrophy, all with −log10(P) > 3. Furthermore, scMetabolism analysis showed a marked decline in glutathione metabolism and mitochondrial ATP production as the disease progressed.
Conclusion: Single-cell transcriptomic and metabolic dynamics profiles suggest that the reduced contractility observed in our DMD cardiomyopathy model is driven by mitochondrial dysfunction caused by increased sensitivity to oxidative and inflammatory stress, as well as microtubule instability. These findings provide novel insights beyond the previously reported membrane fragility due to dystrophin deficiency.
Methods: EHTs were generated from patient iPSC-derived cardiomyocytes and epicardial cell–derived non-cardiomyocytes. An isogenic control with a corrected dystrophin mutation was used as comparison. To induce an adult-like disease phenotype, we shifted cardiomyocyte metabolism toward fatty acid oxidation and applied pathological stress. Single-cell RNA sequencing (scRNA-seq) was conducted before and after pathological stimulation to capture disease-associated molecular changes.
Results: Phenotypic assessment revealed that while control EHTs showed increased contractility during maturation, DMD EHTs lacked this improvement (p < 0.05) and exhibited disorganized sarcomeres (p < 0.01). Upon pathological stimulation with mechanical stress and inflammatory cytokines, DMD EHTs showed a further decline in contractility (p < 0.01), accompanied by increased fibrosis (p < 0.05) and DNA damage. TFvelocity analysis of time-course scRNA-seq revealed that DMD cardiomyocytes exhibited a gene expression profile resembling that of dilated cardiomyopathy. This analysis identified early activation of pathways related to microtubule instability and fibrosis, followed by later activation of pathways associated with responses to oxidative stress and inflammation, as well as muscle atrophy, all with −log10(P) > 3. Furthermore, scMetabolism analysis showed a marked decline in glutathione metabolism and mitochondrial ATP production as the disease progressed.
Conclusion: Single-cell transcriptomic and metabolic dynamics profiles suggest that the reduced contractility observed in our DMD cardiomyopathy model is driven by mitochondrial dysfunction caused by increased sensitivity to oxidative and inflammatory stress, as well as microtubule instability. These findings provide novel insights beyond the previously reported membrane fragility due to dystrophin deficiency.
More abstracts on this topic:
Age Associated T cells (TAA cells) expressing Granzyme k are novel cell types in Atherosclerotic plaques.
Patil Mallikarjun, Tyrrell Daniel, Ali Md Akkas, Siam Md Hasanul Banna, Brazell James
Elucidation of the mechanism of DMD cardiomyopathy with the use of a FRET-based sarcomere activation biosensorMartin Ashley, Metzger Joseph