Final ID: MP1103
The Klotho Protein Reduces Vascular Calcification via Suppressing GPX4-mediated Ferroptosis in Vascular Smooth Muscle Cells
Abstract Body (Do not enter title and authors here): OBJECTIVES
Chronic kidney disease (CKD) patients exhibit elevated cardiovascular risk linked to vascular calcification—pathological calcium-phosphate deposition impairing vascular compliance. Previous studies identified reduced α-Klotho expression in CKD, potentially exacerbating phosphate dyshomeostasis. Hyperphosphatemia promotes vascular calcification by inducing ferroptosis in vascular smooth muscle cells (VSMCs) via GSH/GPX4 pathway suppression. This study aimed to elucidate α-Klotho's role in regulating ferroptosis-dependent calcification.
METHODS
VSMCs were treated with high-phosphate (2.6 mM) medium to mimic CKD calcific stress, with/without recombinant α-Klotho (100 ng/ml). Osteogenic markers (Runx2, OST, ALP) were assessed by western blot. Ferroptosis markers (Ptgs2/COX2, GSH, GPX4) and lipid peroxidation (Liperfluo) were analyzed via RT-qPCR/western blot. Intracellular Fe2+ (FerroOrange) and viability (CCK-8) were quantified. Ferroptosis inducer RSL3 was used for validation.
RESULTS
(1) High-phosphate-induced calcification & ferroptosis were attenuated by α-Klotho:
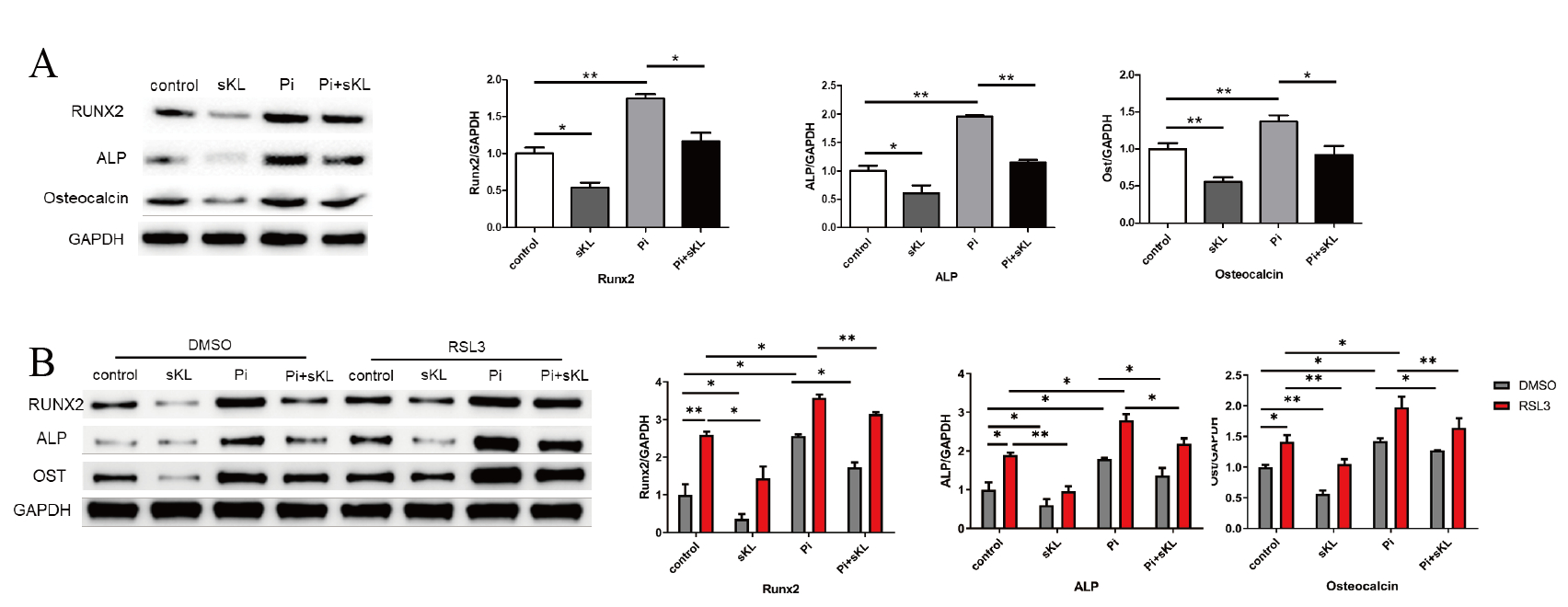
(i) High-phosphate significantly increased osteogenic markers (Runx2, ALP, OCN; P<0.05 vs. control), suppressed by α-Klotho (Fig 1A).
(ii) α-Klotho inhibited RSL3-induced VSMC calcification (Fig 1B).
(2) α-Klotho restored redox homeostasis:
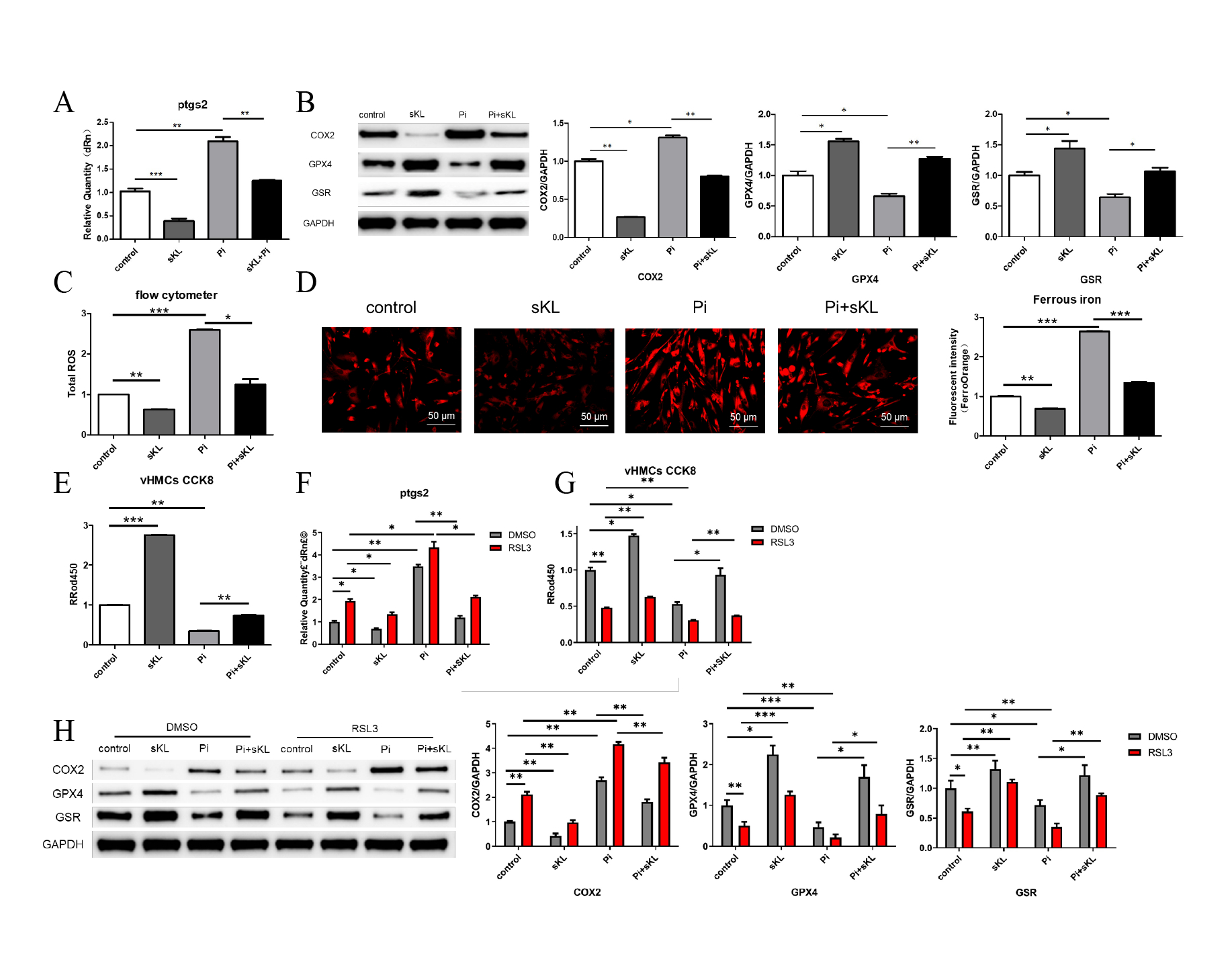
(i) High-phosphate upregulated COX2 (P<0.05) and downregulated GSH/GPX4; α-Klotho reversed these effects (Fig 2A-B).
(ii) High-phosphate increased lipid ROS and Fe2+ accumulation while reducing viability; α-Klotho suppressed lipid ROS/Fe2+ and restored viability (Fig 2C-E).
(iii) α-Klotho blocked RSL3-triggered calcification (Fig 2F-H).
Conclusion: α-Klotho inhibits High-phosphate-driven calcification via dual mechanisms: 1) Reactivating GSH/GPX4 to suppress ferroptosis; 2) Inhibiting VSMC osteogenic transdifferentiation.
CONCLUSIONS
α-Klotho inhibits High-phosphate-induced VSMC ferroptosis by activating GSH/GPX4, reducing lipid ROS/Fe2+ accumulation, and restoring viability. Concurrently, it downregulates osteogenic markers (Runx2/ALP/OCN), preventing phenotypic transition. These findings reveal α-Klotho's dual protection against vascular calcification via coordinated suppression of ferroptosis and osteogenic differentiation (Fig 1-2).
Chronic kidney disease (CKD) patients exhibit elevated cardiovascular risk linked to vascular calcification—pathological calcium-phosphate deposition impairing vascular compliance. Previous studies identified reduced α-Klotho expression in CKD, potentially exacerbating phosphate dyshomeostasis. Hyperphosphatemia promotes vascular calcification by inducing ferroptosis in vascular smooth muscle cells (VSMCs) via GSH/GPX4 pathway suppression. This study aimed to elucidate α-Klotho's role in regulating ferroptosis-dependent calcification.
METHODS
VSMCs were treated with high-phosphate (2.6 mM) medium to mimic CKD calcific stress, with/without recombinant α-Klotho (100 ng/ml). Osteogenic markers (Runx2, OST, ALP) were assessed by western blot. Ferroptosis markers (Ptgs2/COX2, GSH, GPX4) and lipid peroxidation (Liperfluo) were analyzed via RT-qPCR/western blot. Intracellular Fe2+ (FerroOrange) and viability (CCK-8) were quantified. Ferroptosis inducer RSL3 was used for validation.
RESULTS
(1) High-phosphate-induced calcification & ferroptosis were attenuated by α-Klotho:
(i) High-phosphate significantly increased osteogenic markers (Runx2, ALP, OCN; P<0.05 vs. control), suppressed by α-Klotho (Fig 1A).
(ii) α-Klotho inhibited RSL3-induced VSMC calcification (Fig 1B).
(2) α-Klotho restored redox homeostasis:
(i) High-phosphate upregulated COX2 (P<0.05) and downregulated GSH/GPX4; α-Klotho reversed these effects (Fig 2A-B).
(ii) High-phosphate increased lipid ROS and Fe2+ accumulation while reducing viability; α-Klotho suppressed lipid ROS/Fe2+ and restored viability (Fig 2C-E).
(iii) α-Klotho blocked RSL3-triggered calcification (Fig 2F-H).
Conclusion: α-Klotho inhibits High-phosphate-driven calcification via dual mechanisms: 1) Reactivating GSH/GPX4 to suppress ferroptosis; 2) Inhibiting VSMC osteogenic transdifferentiation.
CONCLUSIONS
α-Klotho inhibits High-phosphate-induced VSMC ferroptosis by activating GSH/GPX4, reducing lipid ROS/Fe2+ accumulation, and restoring viability. Concurrently, it downregulates osteogenic markers (Runx2/ALP/OCN), preventing phenotypic transition. These findings reveal α-Klotho's dual protection against vascular calcification via coordinated suppression of ferroptosis and osteogenic differentiation (Fig 1-2).
More abstracts on this topic:
AI-Measured Thoracic Ascending Aortic Calcification in CAC Scans Predicts Cardiovascular Events: An AI-CVD study in the FHS Offspring Cohort
Naghavi Morteza, Atlas Kyle, Zhang Chenyu, Reeves Anthony, Atlas Thomas, Wasserthal Jakob, Wong Nathan, Benjamin Emelia, Levy Daniel
Ambulatory QT Interval Prolongation and Reduced T-Amplitude Help Identify Veterans with Heart DiseaseShah Amit, Colon-motas Kay, De Leon Katalina, Murtala Abdulkareem, Osei Jeffery, Mourad Rama, Chang Sarah, Ko Yi-an, Zeng Zhaohua, Sheikh Shafa-at, Xu Ying, Koscova Zuzana, Kim Kain, Akoto Natalie, Santana Eric, Alexander Abigail, Suvada Kara, Roberts Tatum, Stefanos Lewam, Vaccarino Viola, Clifford Gari, Li Qiao, Sameni Reza, Bahrami Rad Ali, Karimi Sajjad, Lampert Rachel, Mathew Lejy, Alagar Nikila