Final ID: Su4006
Intracellular β1AR-Phosphodiesterases 4D Axis Control Local ER Stress in HFpEF
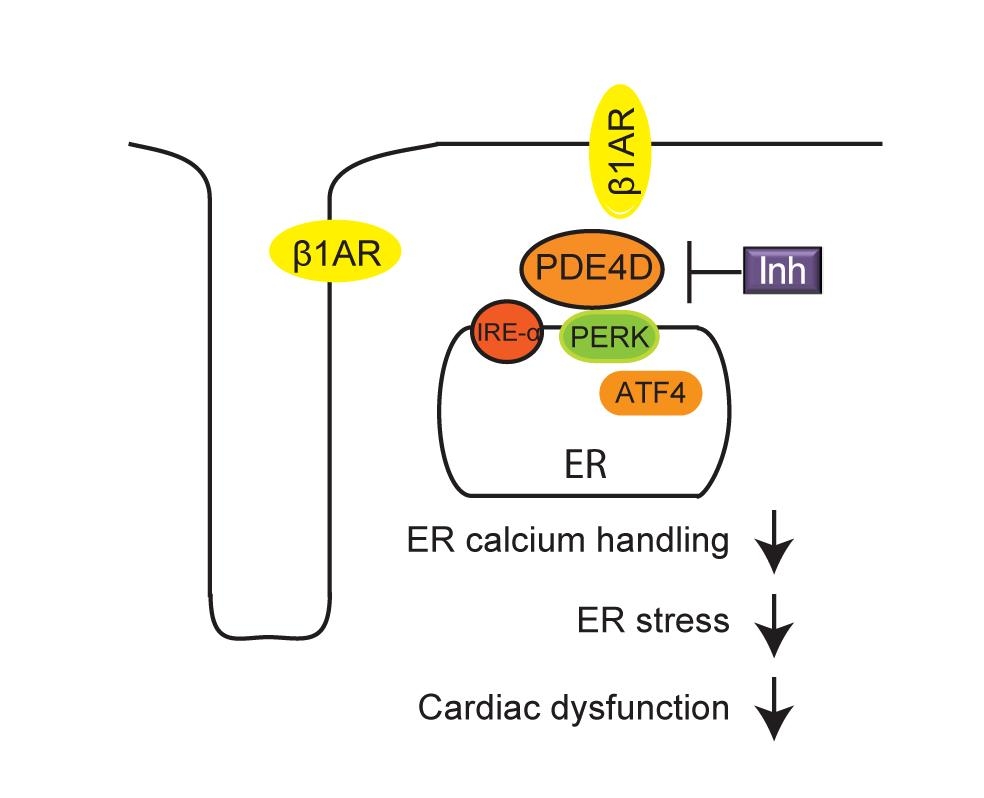
Abstract Body (Do not enter title and authors here): OBJECTIVE: Heart failure with preserved ejection fraction (HFpEF) is a major global health problem with limited therapeutic options. Despite its high prevalence, the molecular mechanisms underlying HFpEF remain poorly understood. We hypothesize that disruption of calcium homeostasis induces endoplasmic reticulum (ER) stress via localized intracellular β-adrenergic receptor (βAR) interaction with phosphodiesterases 4D (PDE4D) in HFpEF.
METHODS AND RESULTS: Wild-type mice were fed a high-fat diet (HFD) and N(ω)-nitro-L-arginine methyl ester (L-NAME) to induce HFpEF. Echocardiography was used to assess diastolic function via the E/E' ratio (early diastolic transmitral flow velocity to early diastolic mitral annular velocity). Treatment with the PDE4D inhibitor Zatolmilast significantly reduced E/E' compared to saline-treated HFpEF controls, indicating improved diastolic function. Sirius Red staining showed marked interstitial and perivascular fibrosis in HFpEF hearts, which was attenuated by Zatolmilast.
Adult cardiomyocytes were isolated from normal chow (NC), HFpEF, and HFpEF+Zatolmilast mice and analyzed using FRET-based PKA biosensors and excitation-contraction coupling (ECC) assays. In HFpEF cardiomyocytes, PDE4D was upregulated and showed increased association with β1AR at the ER/SR, resulting in localized cAMP degradation. PDE4D inhibition restored β1AR-mediated PKA activity and improved calcium handling, as evidenced by shortened decay tau during calcium reuptake.
Moreover, ER stress markers were assessed by qPCR and Western blot. HFpEF hearts displayed elevated PERK, ATF4, and CHOP expression, which were reduced following Zatolmilast treatment. The phosphorylation of IRE-α was increased after PDE4D inhibition. Conversely, PDE4D overexpression alone was sufficient to elevate PERK, ATF4, and CHOP while decreasing IRE-α phosphorylation, implicating PDE4D as a direct modulator of ER stress signaling.
To further validate these findings, we generated cardiac-specific PDE4D knockout (cKO) mice and induced HFpEF. Compared to floxed controls, PDE4D cKO mice showed improved diastolic function (lower E/E’), supporting the therapeutic relevance of PDE4D deletion in HFpEF.
CONCLUSION: These findings identify PDE4D as a key mediator of ER stress and cardiac dysfunction in HFpEF. Inhibition or genetic deletion of cardiac PDE4D alleviates ER stress, improves calcium handling, and restores diastolic function, highlighting PDE4D as a promising therapeutic target in HFpEF.
METHODS AND RESULTS: Wild-type mice were fed a high-fat diet (HFD) and N(ω)-nitro-L-arginine methyl ester (L-NAME) to induce HFpEF. Echocardiography was used to assess diastolic function via the E/E' ratio (early diastolic transmitral flow velocity to early diastolic mitral annular velocity). Treatment with the PDE4D inhibitor Zatolmilast significantly reduced E/E' compared to saline-treated HFpEF controls, indicating improved diastolic function. Sirius Red staining showed marked interstitial and perivascular fibrosis in HFpEF hearts, which was attenuated by Zatolmilast.
Adult cardiomyocytes were isolated from normal chow (NC), HFpEF, and HFpEF+Zatolmilast mice and analyzed using FRET-based PKA biosensors and excitation-contraction coupling (ECC) assays. In HFpEF cardiomyocytes, PDE4D was upregulated and showed increased association with β1AR at the ER/SR, resulting in localized cAMP degradation. PDE4D inhibition restored β1AR-mediated PKA activity and improved calcium handling, as evidenced by shortened decay tau during calcium reuptake.
Moreover, ER stress markers were assessed by qPCR and Western blot. HFpEF hearts displayed elevated PERK, ATF4, and CHOP expression, which were reduced following Zatolmilast treatment. The phosphorylation of IRE-α was increased after PDE4D inhibition. Conversely, PDE4D overexpression alone was sufficient to elevate PERK, ATF4, and CHOP while decreasing IRE-α phosphorylation, implicating PDE4D as a direct modulator of ER stress signaling.
To further validate these findings, we generated cardiac-specific PDE4D knockout (cKO) mice and induced HFpEF. Compared to floxed controls, PDE4D cKO mice showed improved diastolic function (lower E/E’), supporting the therapeutic relevance of PDE4D deletion in HFpEF.
CONCLUSION: These findings identify PDE4D as a key mediator of ER stress and cardiac dysfunction in HFpEF. Inhibition or genetic deletion of cardiac PDE4D alleviates ER stress, improves calcium handling, and restores diastolic function, highlighting PDE4D as a promising therapeutic target in HFpEF.
More abstracts on this topic:
A TTN Frameshift Mutation Leads to Dysfunctional Sarcomere and Calcium Handling in iPSC-derived Atrial Cardiomyocytes
Baskaran Abhinaya, Griza Decebal, Chen Yining, Diaz Annette, Darbar Dawood, Arif Mahmud, Chen Hanna, Hill Michael, Barney Miles, Owais Asia, Sridhar Arvind, Desantiago Jaime, Shah Anish
Fluorescence-Labeled Very Low-Density Lipoproteins (VLDLs) Clearance Method for the Assessment of Dynamic VLDLs Function in MiceZu Yujiao, Pahlavani Mandana, Walker Tatum, Scoggin Shane, Moustaid-moussa Naima