Final ID: MP2515
Effects of Finerenone on Individual Components of the Kansas City Cardiomyopathy Questionnaire in Heart Failure With Mildly Reduced or Preserved Ejection Fraction
Methods: This is a prespecified analysis of the FINEARTS-HF trial, a double-blind randomized comparison of finerenone with placebo in patients with symptomatic HF and left ventricular ejection fraction (LVEF) ≥40%. KCCQ was administered at randomization and at 6, 9, and 12 months. Each of the 23 individual KCCQ components was scaled from 0 (worst symptoms) to 100 (best symptoms). Mean score changes from randomization to 12 months for each 23 KCCQ components were examined using multivariable linear regression models, adjusting for each corresponding baseline KCCQ value.
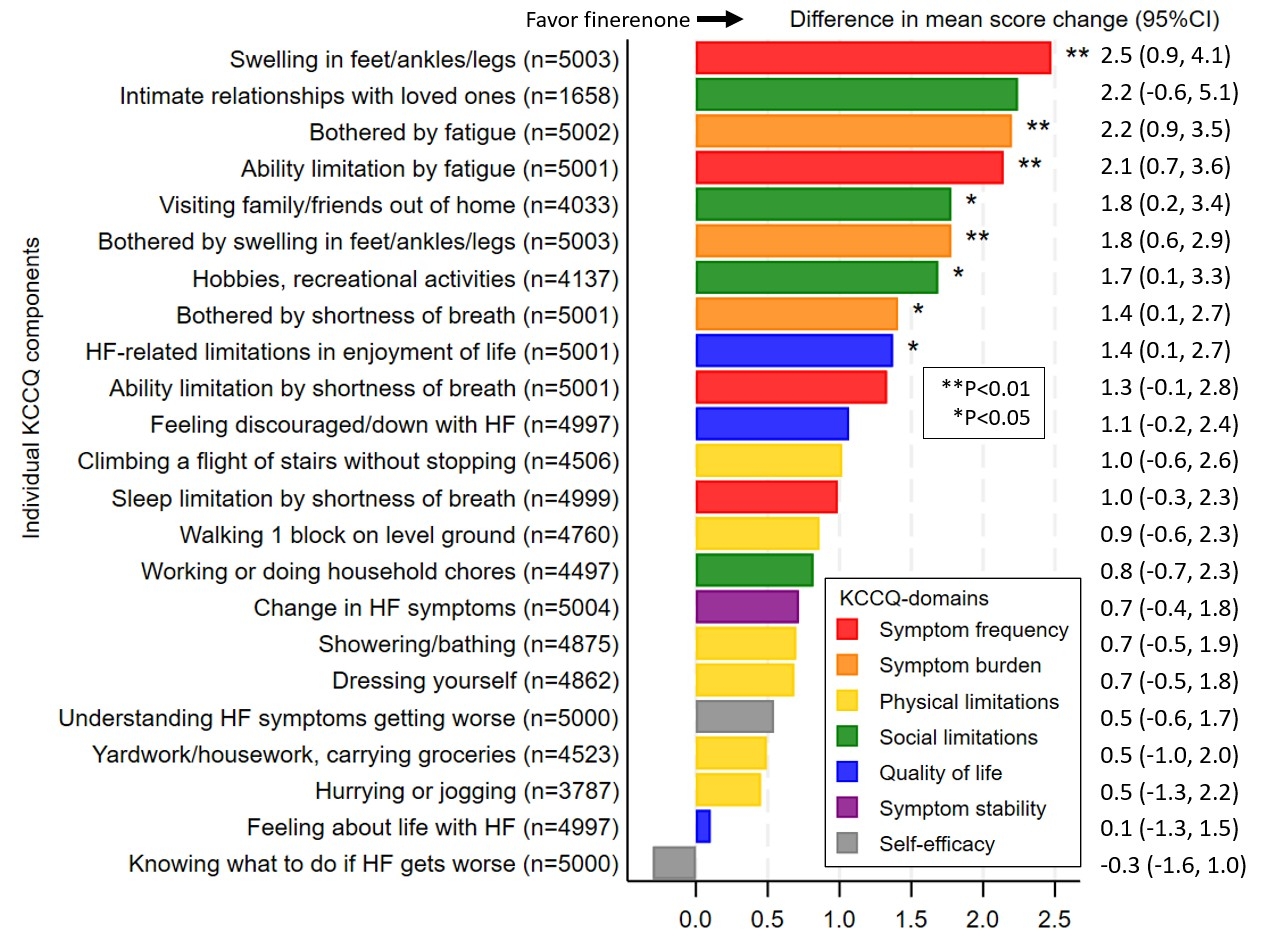
Results: Of the 6,001 randomized participants in the FINEARTS-HF trial, KCCQ data were available for 5,987 at randomization, of whom 5,006 (83% of the overall population) completed KCCQ assessment at 12 months (mean age: 72±10 years, women: 45%, NYHA class II: 71%, and mean LVEF: 53±8%). Among the 23 items of KCCQ, the greatest nominal significant improvements with finerenone were observed in the frequency of lower limb edema (difference: 2.5, 95%CI: 0.9-4.1), burden of fatigue (difference: 2.2, 95%CI: 0.9-3.5), frequency of fatigue (difference: 2.1, 95%CI: 0.7-3.6), and burden of lower limb edema (difference: 1.8, 95%CI: 0.6-2.9) (all P<0.01) (Figure). Longitudinal analyses integrating data from months 6, 9, and 12 were consistent with the main results. The proportion of patients with worsening score from randomization to 12 months was numerically lower with finerenone versus placebo for most individual KCCQ components.
Conclusions: In the FINEARTS-HF trial of patients with HFmrEF/HFpEF, finerenone was associated with improvement in a broad range of individual KCCQ components, with the greatest benefits seen in domains related to symptom frequency, symptom burden, and social limitations.
- Hamatani, Yasuhiro ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Lay-flurrie, James ( Bayer plc , Reading , United Kingdom )
- Lage, Andrea ( Bayer SA , Sao Paulo , Brazil )
- Hofmeister, Lucas ( Bayer AG , Berlin , Germany )
- Mcmurray, John ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Peikert, Alexander ( University Heart Center Graz , Graz , Austria )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
- Senni, Michele ( ASST PAPA GIOVANNI XXIII , Bergamo , Italy )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Voors, Adriaan ( UNIVERSITY MEDICAL CENTER GRONINGEN , Gronien , Netherlands )
Meeting Info:
Session Info:
(Non-) Roid Rage: Novel Insights Into Non-Steroidal MRAs for HF
Monday, 11/10/2025 , 12:15PM - 01:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Ocaranza Maria Paz, Jimenez Veronica, Yanez Osvaldo, Jalil Jorge, Venegas Camilo, Candia Camila, Hermoso Marcela, Gabrielli Luigi, Morales Javier, Oyarzun Felipe, Torres Cristian, Lillo Pablo
Association Between Postprandial Hypotension Determined by Ambulatory Blood Pressure Monitoring and Falls Among Older Adults with Hypertension Who Are Taking Antihypertensive Medication: Results from the AMBROSIA StudyNarita Keisuke, Schwartz Joseph, Sim John, Shimbo Daichi, Reynolds Kristi, Wei Rong, Harrison Teresa, Cannavale Kimberly, Qian Lei, Bowling Barrett, Fang Chloe, Muntner Paul
More abstracts from these authors:
Chimura Misato, Senni Michele, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Lay-flurrie James, Scalise Andrea, Rohwedder Katja, Lam Carolyn
Effect of finerenone on a hierarchical composite endpoint analyzed using win statistics in patients with heart failure and mildly reduced or preserved ejection fraction: A prespecified analysis of FINEARTS-HF.Kondo Toru, Amarante Flaviana, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Brinker Meike, Lay-flurrie James, Schloemer Patrick, Viswanathan Prabhakar