Final ID: MP1088
Fibroblast Progenitor Cells Contribute to Vasculogenesis During Regenerative Wound Healing
Abstract Body (Do not enter title and authors here): Background
The goal of tissue regeneration is to restore structure and function. In adult regenerative wound healing, dermal fibroblasts exhibit multipotency and can be reprogrammed into lineages such as hair follicle cells and adipocytes, etc. Neural cell adhesion molecule 1 (NCAM1), a membrane-bound adhesion protein expressed in dermal fibroblasts, plays a key role in cell-cell and cell-matrix interactions and has been implicated in fate transitions during tissue remodeling. NCAM1 is absent in normal endothelium but is aberrantly expressed in tumor-derived endothelial cells, where it promotes capillary morphogenesis. These observations suggest that NCAM1+ fibroblasts may represent a progenitor-like state more capable of endothelial conversion than NCAM1- cells. While angiogenesis is the main vascularization process in adult wounds, vasculogenesis from progenitors like fibroblasts may also contribute. Fibroblast-to-endothelial reprogramming has been demonstrated in vitro using defined reprogramming factors, but its in vivo evidence remains to be investigated. We hypothesized that NCAM1+ dermal fibroblasts give rise to a subset of regenerated endothelial cells.
Aims
(1) Characterize vasculature formation during regenerative wound healing
(2) Identify progenitor populations and reprogramming cues, including transcription factors and adhesion molecules
Method
Using a wound-induced hair neogenesis (WIHN) mouse model, we performed scRNA sequencing and RNA velocity analyses on post-wounding day 14. Wholemount immunostaining assessed vascular morphology and cell identity. NCAM1-CreERT2×ROSA26 reporter mice for lineage tracing.
Results
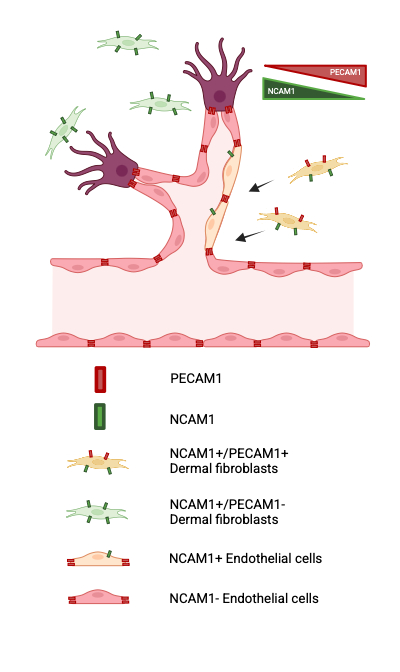
The regenerated wound bed showed two contrasting vasculature patterns: disrupted, discontinuous vessels in the regenerating wound center and organized vasculature in the wound margin. scRNA-seq and velocity analyses indicated PECAM1+ cells may originate from NCAM1+ fibroblast-like cells that also expressed fibroblast-to-endothelial cells reprogramming factors such as FOXO1, TAL1 and SOX17. Wholemount staining revealed individual cells and a fraction of endothelial cells co-expressing PECAM1, PDGFRα, and NCAM1 in the capillary-like vasculature in wound dermis.
Conclusion
Our findings support vasculogenesis during regenerative wound healing, with NCAM1+ fibroblasts contributing to endothelial cell populations. These results provide new insights into fibroblast-endothelial plasticity and vascular regeneration in adult tissue regeneration.
The goal of tissue regeneration is to restore structure and function. In adult regenerative wound healing, dermal fibroblasts exhibit multipotency and can be reprogrammed into lineages such as hair follicle cells and adipocytes, etc. Neural cell adhesion molecule 1 (NCAM1), a membrane-bound adhesion protein expressed in dermal fibroblasts, plays a key role in cell-cell and cell-matrix interactions and has been implicated in fate transitions during tissue remodeling. NCAM1 is absent in normal endothelium but is aberrantly expressed in tumor-derived endothelial cells, where it promotes capillary morphogenesis. These observations suggest that NCAM1+ fibroblasts may represent a progenitor-like state more capable of endothelial conversion than NCAM1- cells. While angiogenesis is the main vascularization process in adult wounds, vasculogenesis from progenitors like fibroblasts may also contribute. Fibroblast-to-endothelial reprogramming has been demonstrated in vitro using defined reprogramming factors, but its in vivo evidence remains to be investigated. We hypothesized that NCAM1+ dermal fibroblasts give rise to a subset of regenerated endothelial cells.
Aims
(1) Characterize vasculature formation during regenerative wound healing
(2) Identify progenitor populations and reprogramming cues, including transcription factors and adhesion molecules
Method
Using a wound-induced hair neogenesis (WIHN) mouse model, we performed scRNA sequencing and RNA velocity analyses on post-wounding day 14. Wholemount immunostaining assessed vascular morphology and cell identity. NCAM1-CreERT2×ROSA26 reporter mice for lineage tracing.
Results
The regenerated wound bed showed two contrasting vasculature patterns: disrupted, discontinuous vessels in the regenerating wound center and organized vasculature in the wound margin. scRNA-seq and velocity analyses indicated PECAM1+ cells may originate from NCAM1+ fibroblast-like cells that also expressed fibroblast-to-endothelial cells reprogramming factors such as FOXO1, TAL1 and SOX17. Wholemount staining revealed individual cells and a fraction of endothelial cells co-expressing PECAM1, PDGFRα, and NCAM1 in the capillary-like vasculature in wound dermis.
Conclusion
Our findings support vasculogenesis during regenerative wound healing, with NCAM1+ fibroblasts contributing to endothelial cell populations. These results provide new insights into fibroblast-endothelial plasticity and vascular regeneration in adult tissue regeneration.
More abstracts on this topic:
First-in-human Phase 1/2a Study of Intracerebral Transplantation using Embryonic-derived Neural Stem Cells (NR1) for Chronic Ischemic Stroke (NCT04631406): 12 Months Outcomes
Steinberg Gary, Jiang Bin, Tong Elizabeth, Qin Feifei, Han Summer, Schwartz Neil, Bet Anthony, Williams Jennifer, Mcdonald Kathy, J Diaz Robert, Harryman Samos Cindy, Trisler Kirk, Weissinger Judy, Coburn Maria
“Hypoxia impairs cardiac organoid contractility in a multi-cell type model of Ischemia-Reperfusion Injury.”Heathershaw Caleb, Shakeriastani Kiarash, Parsons Megan, Latham Olivia, Diaz Jonathan, Maxwell Joshua