Final ID: Mo4008
Biogenic RYR2 Loss-of-Function Disrupts Intracellular Calcium Handling and Induces Electrical Remodeling in Re-Engineered Human Cardiomyocytes
Abstract Body (Do not enter title and authors here): Background: Calcium release channel deficiency syndrome (CRCDS) is caused by biogenic or biophysical loss-of-function (LOF) pathogenic variants in the RYR2-encoded ryanodine receptor (RyR2), a key intracellular Ca2+ release channel. Previously, we identified a novel homozygous duplication involving the promoter and exons 1-4 of RYR2, leading to 80% RyR2 protein loss and exertion-related sudden death in the young. Here, we generated a RYR2 knockout (RYR2-KO) induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM) model to explore intracellular and electrophysiological compensatory mechanisms that overcome this extreme loss of RyR2 protein.
Methods: Using CRISPR/Cas9 gene editing, a homozygous c.163delT variant (p.S55Pfs*46) was inserted into a normal iPSC line (isogenic control) to create a homozygous RYR2-KO iPSC line. After re-engineering the lines into ventricular-like cardiomyocytes (iPSC-CMs), intracellular Ca2+ handling was assessed by Fluo-4 AM cell imaging (0.5 Hz stimulation). Electrical remodeling in the L-type Ca2+ current (ICaL) was assessed using the whole-cell patch clamp technique.
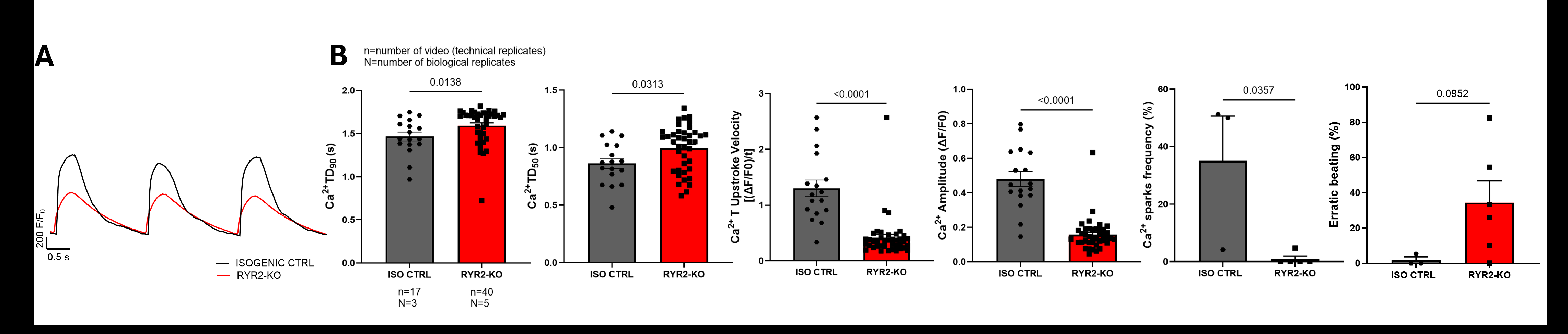
Results: Significant differences were observed in Ca2+ transient parameters between RYR2-KO and isogenic control iPSC-CMs. Biogenic RyR2 loss significantly reduced Ca2+ transient peak amplitude (CTA: 0.16 ± 0.01 ΔF/F0, p<0.0001) and upstroke velocity (CTV: 0.42 ± 0.06 (ΔF/F0)/t, p<0.0001), and prolonged Ca2+ transient duration (CTD90: 1.59 ± 0.03 s, p=0.01) as compared to isogenic control (CTA: 0.48 ± 0.04 ΔF/F0; CTV: 1.30 ± 0.15 (ΔF/F0)/t; CTD90: 1.46 ± 0.05 s). Additionally, RyR2 loss abolished SR Ca2+ leak (0.9 ± 0.9 % versus 35.1 ± 15.5 %, p=0.03). Lastly, a significant reduction in ICaL density was observed in RYR2-KO iPSC-CMs as compared to isogenic control iPSC-CMs (at 0 mV, RYR2-KO: -8.54 ± 1.60 pA/pF, control: -17.45 ± 3.69 pA/pF, p=0.0004). These data indicate that ICaL reduction may contribute to the reduced Ca2+ transient peak.
Conclusions: Complete loss of RyR2 in iPSC-CMs profoundly disrupts intracellular Ca2+ handling, abolishes Ca2+ sparks frequency, and secondarily down-regulates the sarcolemmal L-type Ca2+ channel.
Methods: Using CRISPR/Cas9 gene editing, a homozygous c.163delT variant (p.S55Pfs*46) was inserted into a normal iPSC line (isogenic control) to create a homozygous RYR2-KO iPSC line. After re-engineering the lines into ventricular-like cardiomyocytes (iPSC-CMs), intracellular Ca2+ handling was assessed by Fluo-4 AM cell imaging (0.5 Hz stimulation). Electrical remodeling in the L-type Ca2+ current (ICaL) was assessed using the whole-cell patch clamp technique.
Results: Significant differences were observed in Ca2+ transient parameters between RYR2-KO and isogenic control iPSC-CMs. Biogenic RyR2 loss significantly reduced Ca2+ transient peak amplitude (CTA: 0.16 ± 0.01 ΔF/F0, p<0.0001) and upstroke velocity (CTV: 0.42 ± 0.06 (ΔF/F0)/t, p<0.0001), and prolonged Ca2+ transient duration (CTD90: 1.59 ± 0.03 s, p=0.01) as compared to isogenic control (CTA: 0.48 ± 0.04 ΔF/F0; CTV: 1.30 ± 0.15 (ΔF/F0)/t; CTD90: 1.46 ± 0.05 s). Additionally, RyR2 loss abolished SR Ca2+ leak (0.9 ± 0.9 % versus 35.1 ± 15.5 %, p=0.03). Lastly, a significant reduction in ICaL density was observed in RYR2-KO iPSC-CMs as compared to isogenic control iPSC-CMs (at 0 mV, RYR2-KO: -8.54 ± 1.60 pA/pF, control: -17.45 ± 3.69 pA/pF, p=0.0004). These data indicate that ICaL reduction may contribute to the reduced Ca2+ transient peak.
Conclusions: Complete loss of RyR2 in iPSC-CMs profoundly disrupts intracellular Ca2+ handling, abolishes Ca2+ sparks frequency, and secondarily down-regulates the sarcolemmal L-type Ca2+ channel.