Final ID: MP2680
SSC5D Exacerbates Post-Myocardial Infarction Cardiac Dysfunction via p38/MAPK-Mediated Inflammatory Activation
Abstract Body (Do not enter title and authors here): Background: Myocardial infarction (MI) elicits a robust inflammatory response that is essential for myocardial repair but can also lead to adverse remodeling when excessive. Scavenger receptor cysteine-rich domain-containing protein 5 (SSC5D) has been implicated in various immune responses, but its role in post-MI inflammation remains unclear.
Aims: This study aims to investigate the function and mechanism of macrophage-expressed SSC5D in post-MI inflammation and its impact on cardiac function.
Methods: Peripheral blood was collected from patients with acute coronary syndrome (ACS) and healthy controls to measure serum SSC5D levels. Male C57BL/6J wild-type (WT) and SSC5D knockout (KO) mice (8-10 weeks old) were used. MI was established by the permanent ligation of the left anterior descending coronary artery. An adenoviral vector encoding mouse SSC5D (Ad-SSC5D) was constructed for SSC5D overexpression. Primary macrophages isolated from WT and KO mice were stimulated with lipopolysaccharide (LPS) and interferon (IFN). RNA sequencing (RNA-seq) was performed to identify differentially expressed genes (DEGs) in myocardial tissues. Infarct size, cardiac function, and inflammatory responses were evaluated using histological analysis, echocardiography, western blot, and RT-qPCR.
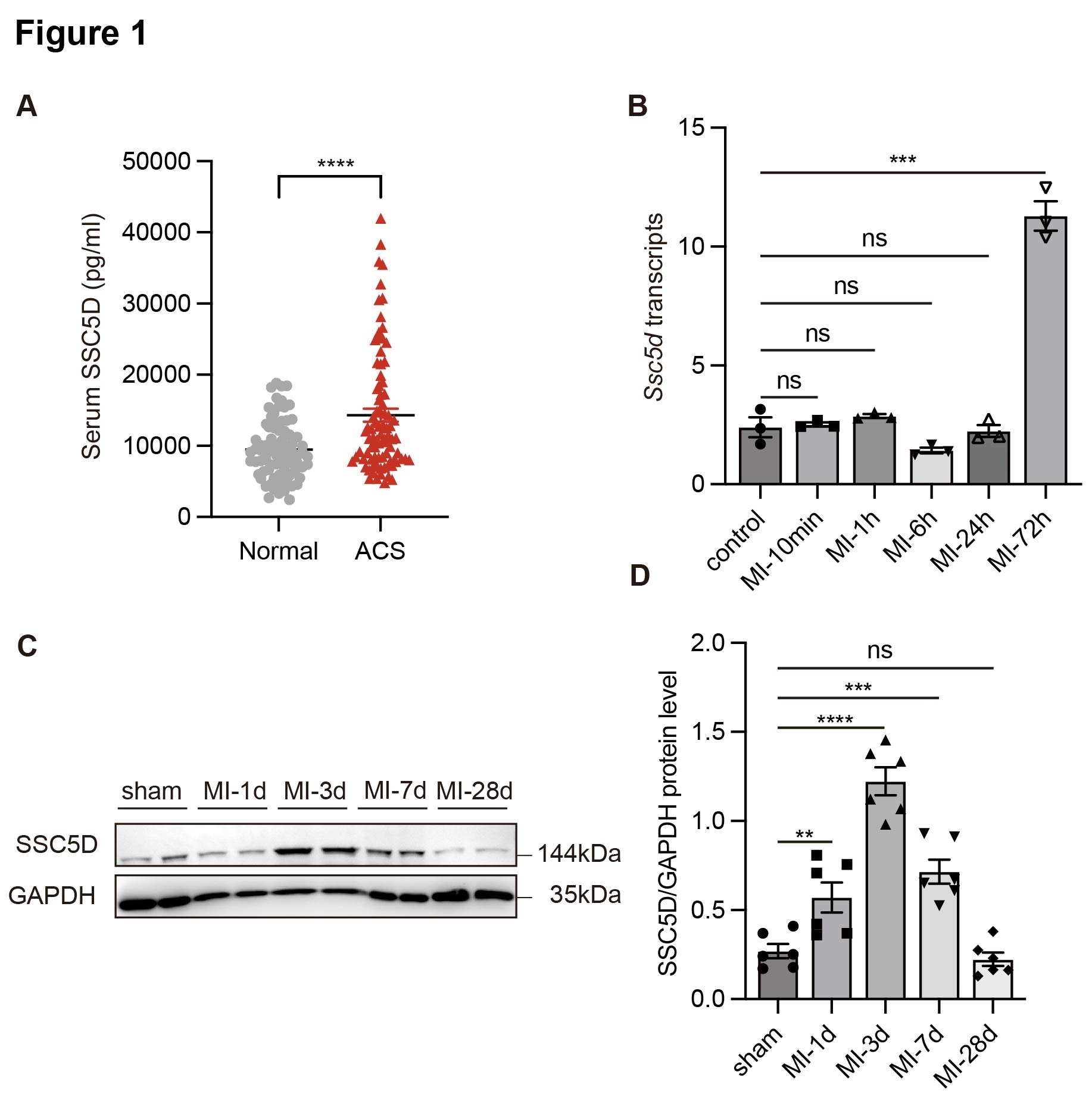
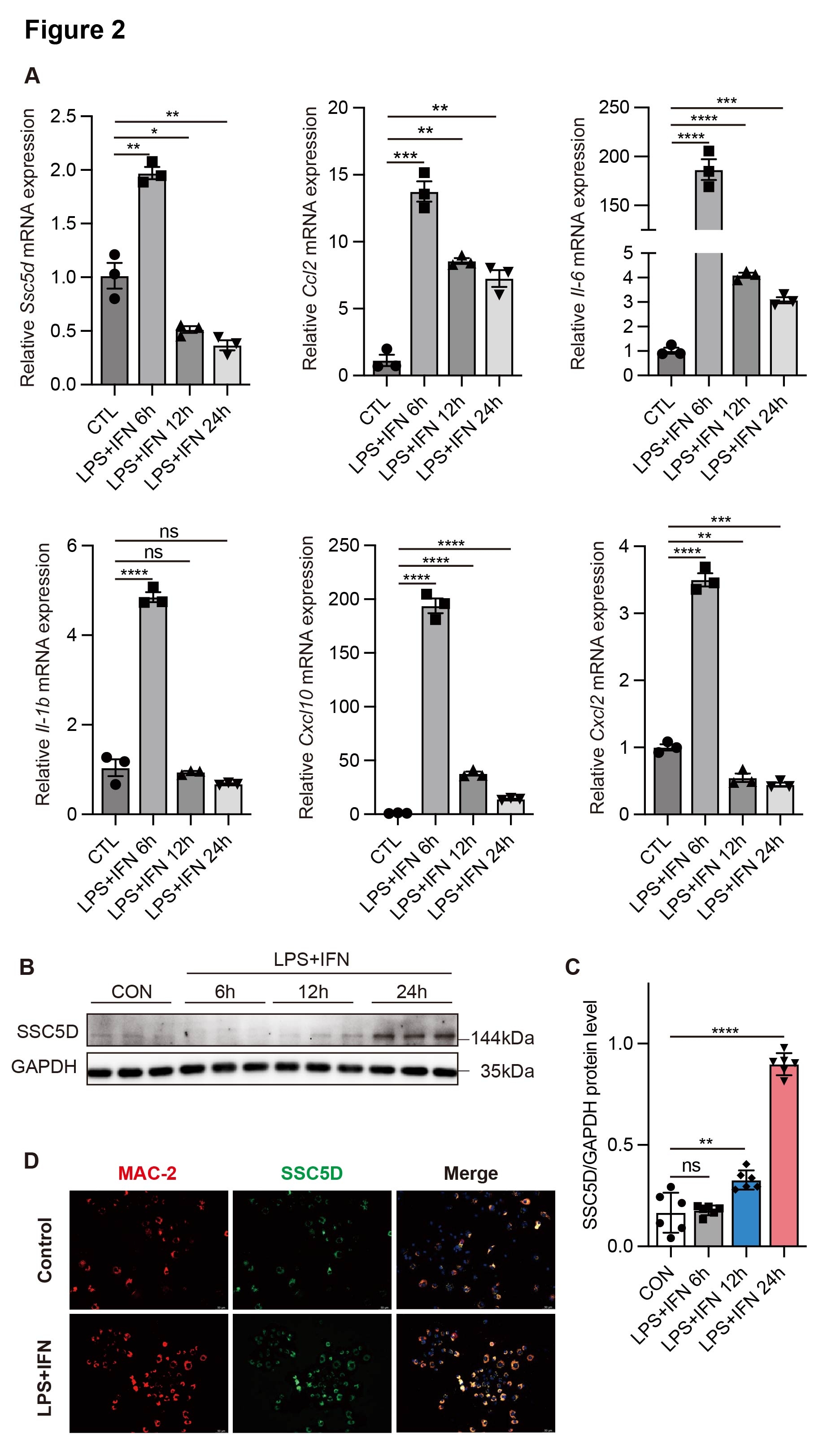
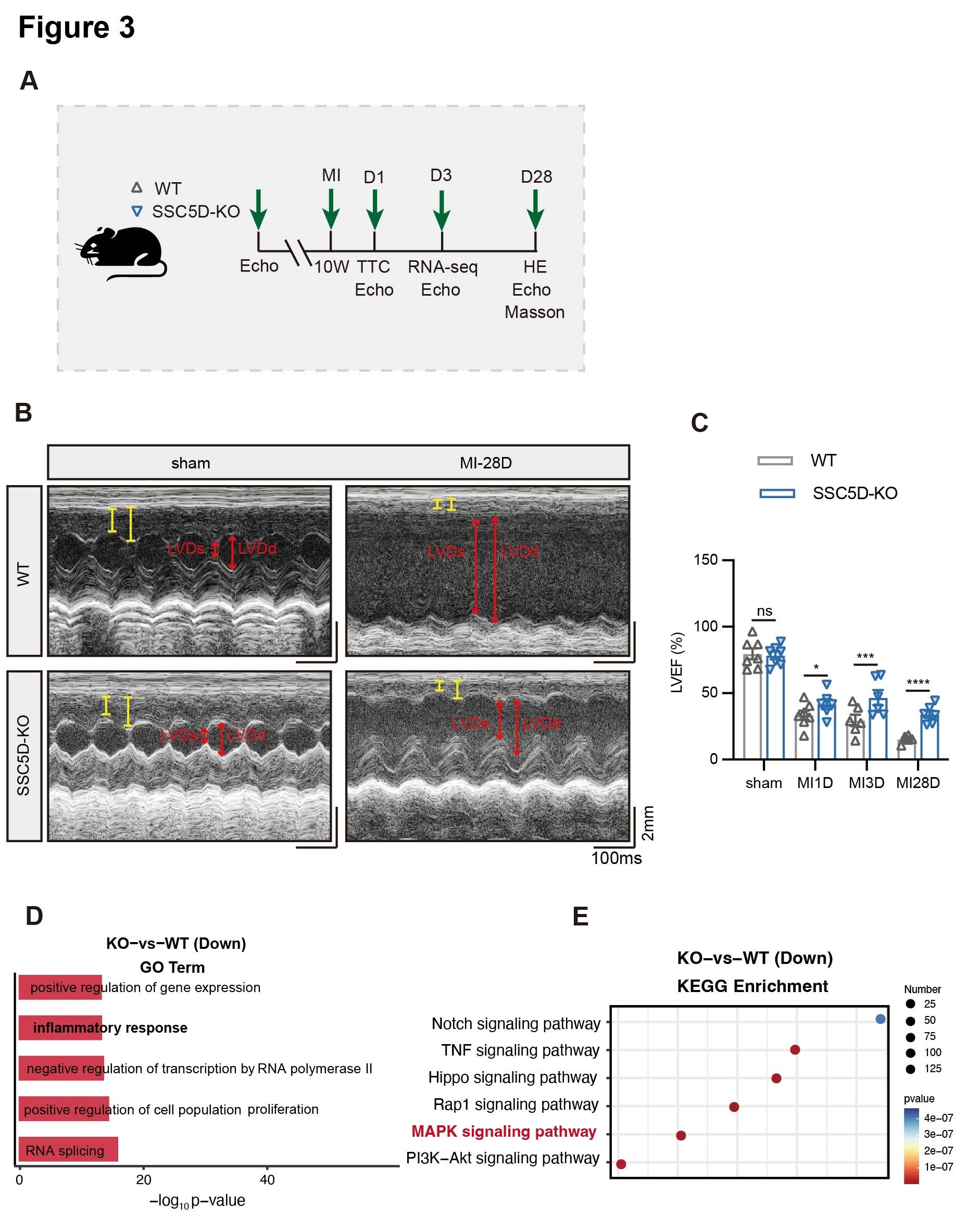
Results: RNA-seq analysis demonstrated that SSC5D was significantly elevated in infarcted myocardial tissues, and serum levels were remarkably upregulated in ACS patients compared to controls. LPS and IFN stimulation significantly upregulated both SSC5D mRNA (2.0 ± 0.4-fold, p < 0.01) and protein expression (5.3 ± 0.3-fold, p < 0.0001) in primary bone marrow-derived macrophages (BMDMs) compared to untreated controls. Knockout of SSC5D remarkably reduced the expression of inflammation-related genes and improved left ventricular ejection fraction (LVEF). Mechanistically, based on the KEGG signaling pathway, we hypothesized that SSC5D promoted the inflammatory response after MI and aggravated heart failure by activating the p38/MAPK signaling pathway.
Conclusions: Our findings demonstrate that macrophage-derived SSC5D plays a critical role in the post-MI inflammatory response by regulating the p38/MAPK signaling pathway. Targeting SSC5D may represent a novel therapeutic strategy to attenuate post-MI inflammation and enhance cardiac recovery.
Aims: This study aims to investigate the function and mechanism of macrophage-expressed SSC5D in post-MI inflammation and its impact on cardiac function.
Methods: Peripheral blood was collected from patients with acute coronary syndrome (ACS) and healthy controls to measure serum SSC5D levels. Male C57BL/6J wild-type (WT) and SSC5D knockout (KO) mice (8-10 weeks old) were used. MI was established by the permanent ligation of the left anterior descending coronary artery. An adenoviral vector encoding mouse SSC5D (Ad-SSC5D) was constructed for SSC5D overexpression. Primary macrophages isolated from WT and KO mice were stimulated with lipopolysaccharide (LPS) and interferon (IFN). RNA sequencing (RNA-seq) was performed to identify differentially expressed genes (DEGs) in myocardial tissues. Infarct size, cardiac function, and inflammatory responses were evaluated using histological analysis, echocardiography, western blot, and RT-qPCR.
Results: RNA-seq analysis demonstrated that SSC5D was significantly elevated in infarcted myocardial tissues, and serum levels were remarkably upregulated in ACS patients compared to controls. LPS and IFN stimulation significantly upregulated both SSC5D mRNA (2.0 ± 0.4-fold, p < 0.01) and protein expression (5.3 ± 0.3-fold, p < 0.0001) in primary bone marrow-derived macrophages (BMDMs) compared to untreated controls. Knockout of SSC5D remarkably reduced the expression of inflammation-related genes and improved left ventricular ejection fraction (LVEF). Mechanistically, based on the KEGG signaling pathway, we hypothesized that SSC5D promoted the inflammatory response after MI and aggravated heart failure by activating the p38/MAPK signaling pathway.
Conclusions: Our findings demonstrate that macrophage-derived SSC5D plays a critical role in the post-MI inflammatory response by regulating the p38/MAPK signaling pathway. Targeting SSC5D may represent a novel therapeutic strategy to attenuate post-MI inflammation and enhance cardiac recovery.
More abstracts on this topic:
β1 Adrenergic Receptor Autoantibodies Promote Heart Failure Though Activation of Prostaglandin E2 Receptor EP1/Phosphodiesterase 4B Pathway
Cao Ning, Qiu Hui, Li Hongwei
4-Hydroxy-2-Nonenal Alters Alternative Polyadenylation to Regulate mRNA Isoform Diversity in the Transition from Human Cardiac Fibroblasts to MyofibroblastsNatarajan Kartiga, Neupane Rahul, Yalamanchili Hari Krishna, Palaniyandi Suresh, Wagner Eric, Guha Ashrith, Amirthalingam Thandavarayan Rajarajan