Final ID: 4363113
A Systems Biology–Driven Strategy to ‘Defat’ Lipid-Associated Macrophages and Reverse Atherosclerosis
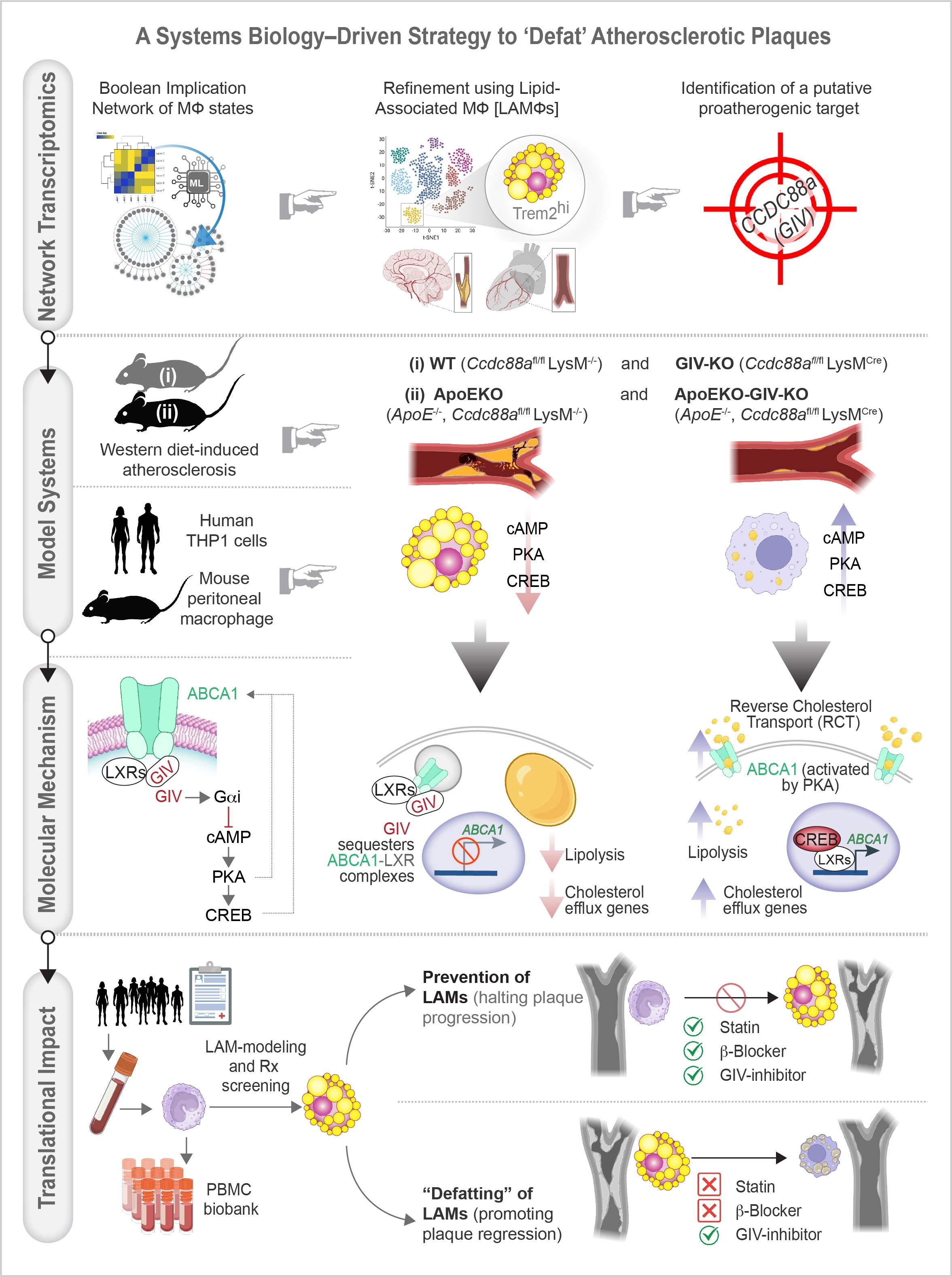
Abstract Body (Do not enter title and authors here): Introduction: Current atherosclerosis therapies like statins lower LDL cholesterol, but they do not reverse established plaques. Even with aggressive LDL-lowering, residual risk persists, driven in part by immunologic factors. A key player in these processes is lipid-associated macrophages (LAM), which accumulate lipids and drive inflammation and plaque instability. Despite their central role, LAM remain un-targetable and here we tackle their untapped therapeutic potential.
Methods: We used a systems biology–driven network transcriptomics approach to identify key myeloid modulators of atherosclerosis. Computational analyses identified CCDC88A, which encodes the cAMP-inhibitor GIV, as a proatherogenic factor. This finding was validated by in vivo and in vitro studies. Our study includes two mouse models: myeloid-specific GIV knockout (MacGIV-KO) and ApoE-deficient MacGIV-KO (n = 8–15, both sexes). Mice were on a Western diet for 12 wks; aortic plaque burden was assessed using Oil Red O staining. For in vitro study, murine peritoneal and THP1-derived macrophages were treated with oxLDL to assess LAM formation, cholesterol uptake/efflux and lipolysis. RNA-seq was used to identify genes in cholesterol metabolism and efflux. Translational relevance was assessed using human PBMCs and a small-molecule inhibitor targeting the GIV/cAMP pathway.
Results: Compared to WT and ApoE-KO controls, GIV-KO reduces aortic plaque burden by 70% and 40%, respectively. Macrophage GIV deficiency inhibits LAM formation, increases cholesterol efflux and basal lipolysis, and is associated with increased mRNA expression of the ‘gatekeeper’ of reverse cholesterol transport (RCT) from peripheral tissues (Abca1) and its transcriptional regulators (LXRα/β). Mechanistically, GIV activates Gαi/βγ proteins and inhibits a well-established anti-atherogenic cyclic AMP (cAMP). GIV binds and sequesters ABCA1 on the endomembrane, suppressing cholesterol efflux. GIV-KO raises cAMP levels, positions ABCA1 at the cell surface and unleashes its activity via a 2-pronged mechanism—both converging on the GIV/cAMP axis: (1) transcriptional activation (CREB), and (2) post-translational modulation via PKA. Small-molecule inhibitors of the GIV/cAMP axis reverse LAMs in both murine and human macrophages, where statins and β-blockers fail.
Conclusion: This study reveals a therapeutic strategy to augment RCT via ABCA1, ‘defat’ LAM and regress plaques, offering a novel approach to treat advanced atherosclerosis.
Methods: We used a systems biology–driven network transcriptomics approach to identify key myeloid modulators of atherosclerosis. Computational analyses identified CCDC88A, which encodes the cAMP-inhibitor GIV, as a proatherogenic factor. This finding was validated by in vivo and in vitro studies. Our study includes two mouse models: myeloid-specific GIV knockout (MacGIV-KO) and ApoE-deficient MacGIV-KO (n = 8–15, both sexes). Mice were on a Western diet for 12 wks; aortic plaque burden was assessed using Oil Red O staining. For in vitro study, murine peritoneal and THP1-derived macrophages were treated with oxLDL to assess LAM formation, cholesterol uptake/efflux and lipolysis. RNA-seq was used to identify genes in cholesterol metabolism and efflux. Translational relevance was assessed using human PBMCs and a small-molecule inhibitor targeting the GIV/cAMP pathway.
Results: Compared to WT and ApoE-KO controls, GIV-KO reduces aortic plaque burden by 70% and 40%, respectively. Macrophage GIV deficiency inhibits LAM formation, increases cholesterol efflux and basal lipolysis, and is associated with increased mRNA expression of the ‘gatekeeper’ of reverse cholesterol transport (RCT) from peripheral tissues (Abca1) and its transcriptional regulators (LXRα/β). Mechanistically, GIV activates Gαi/βγ proteins and inhibits a well-established anti-atherogenic cyclic AMP (cAMP). GIV binds and sequesters ABCA1 on the endomembrane, suppressing cholesterol efflux. GIV-KO raises cAMP levels, positions ABCA1 at the cell surface and unleashes its activity via a 2-pronged mechanism—both converging on the GIV/cAMP axis: (1) transcriptional activation (CREB), and (2) post-translational modulation via PKA. Small-molecule inhibitors of the GIV/cAMP axis reverse LAMs in both murine and human macrophages, where statins and β-blockers fail.
Conclusion: This study reveals a therapeutic strategy to augment RCT via ABCA1, ‘defat’ LAM and regress plaques, offering a novel approach to treat advanced atherosclerosis.
More abstracts on this topic:
A Loss of Function Polymorphism in the Propeptide of Lysyl Oxidase Exacerbates Atherosclerosis
Jung In-hyuk, Amrute Junedh, Luna Sophia, Wagoner Ryan, Lee Paul, Burks Kendall, Holloway Karyn, Alisio Arturo, Stitziel Nathan
A 3-Year, Pre-Trial, Real-world Data Analysis of Patients Enrolled in VICTORION-INITIATE: Insights Using TokenizationRodriguez Fatima, Cosmatos Irene, Desai Nihar, Wright R, Ross Elsie, Ali Yousuf, Kumar Biswajit, Han Guangyang, Cai Beilei, Abbas Cheryl, Ryan Amy