Final ID: Su3128
Reduced occludin protein expression in aortic endothelial cells mediates the mechanism of endothelial barrier dysfunction in aortic dissection disease
Abstract Body (Do not enter title and authors here): Background
Aortic dissection (AD) remains a significant challenge in cardiovascular medicine. Previous studies have found that reduced endothelial cell tight junction ZO-1 protein expression is associated with aortic dissection disease, but it is not clear whether the pattern of Occludin protein expression is the same.
Aims
The aim of this study was to investigate the potential role of aortic endothelial cell Occludin protein in aortic dissection.
Methods
Single-cell RNA sequencing was performed on the ascending aorta of five patients with AD and three heart transplant donors. Additionally, Crispr-cas9 technology and adeno-associated virus 9 (AAV9) were used to generate mouse models with endothelial cell-specific knockout and overexpression of the Ocln gene. Exploration and validation of transcription factors regulating the OCLN gene by dual luciferase gene reporter assay and yeast one-hybrid assay.
Results
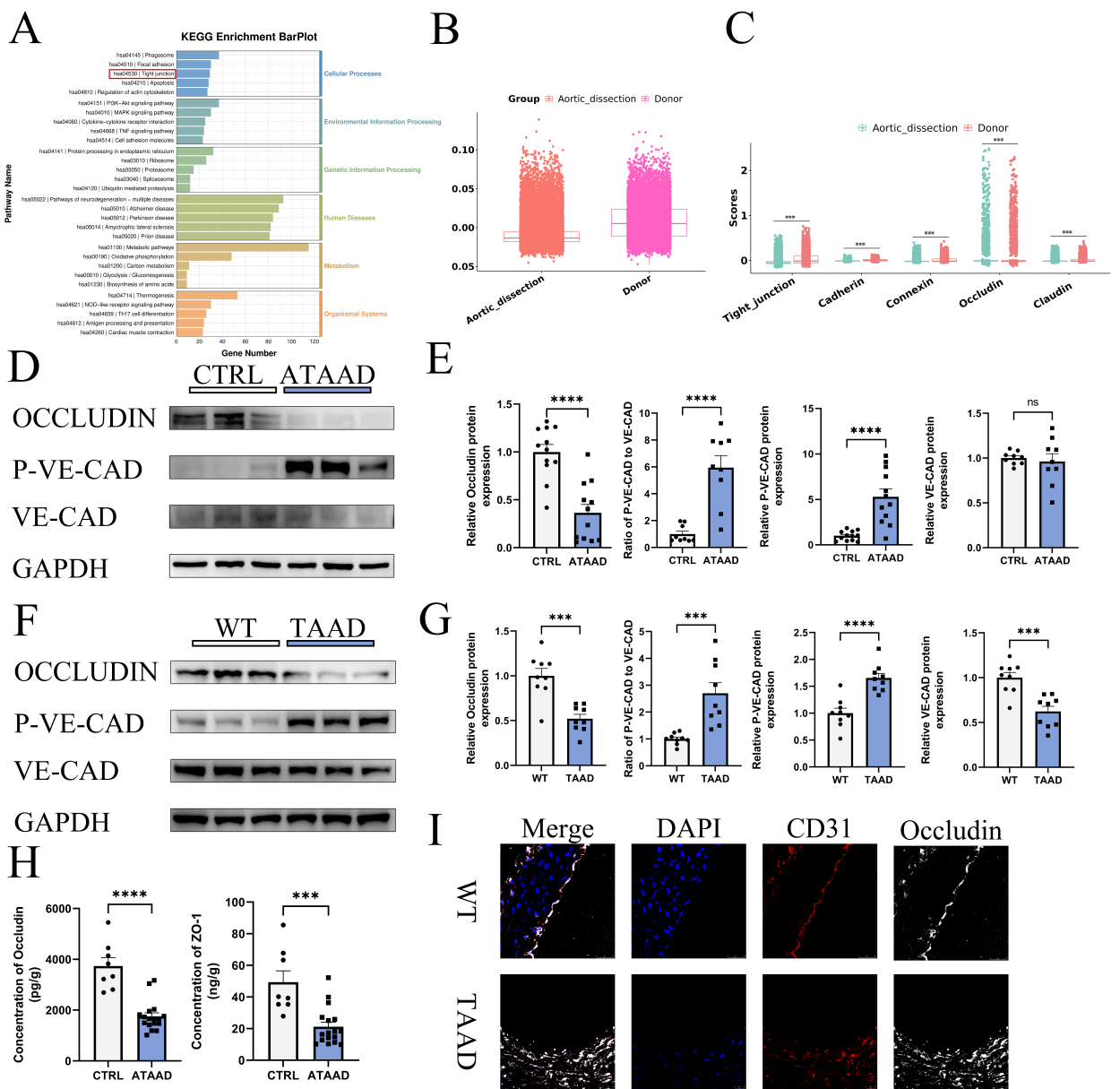
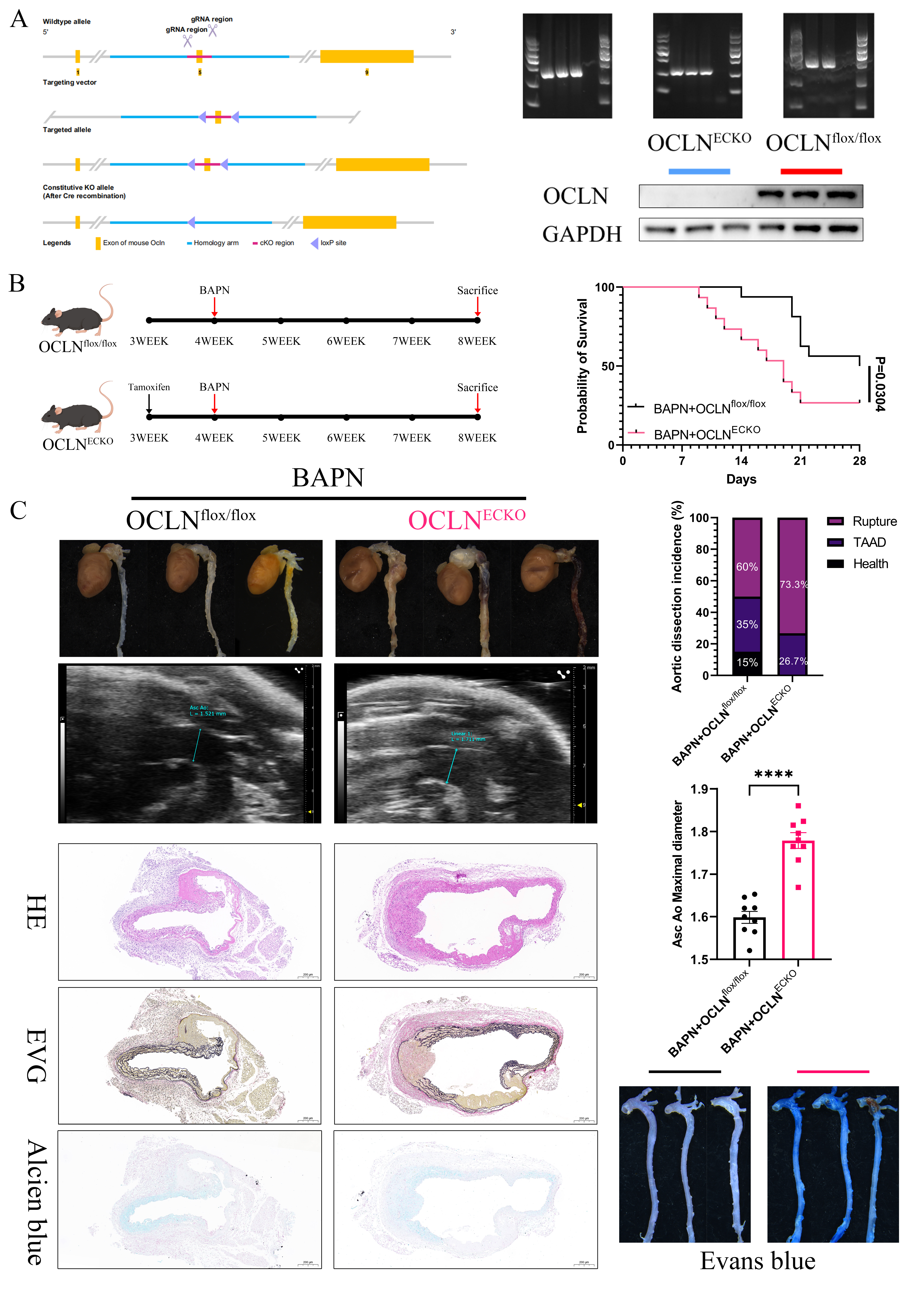
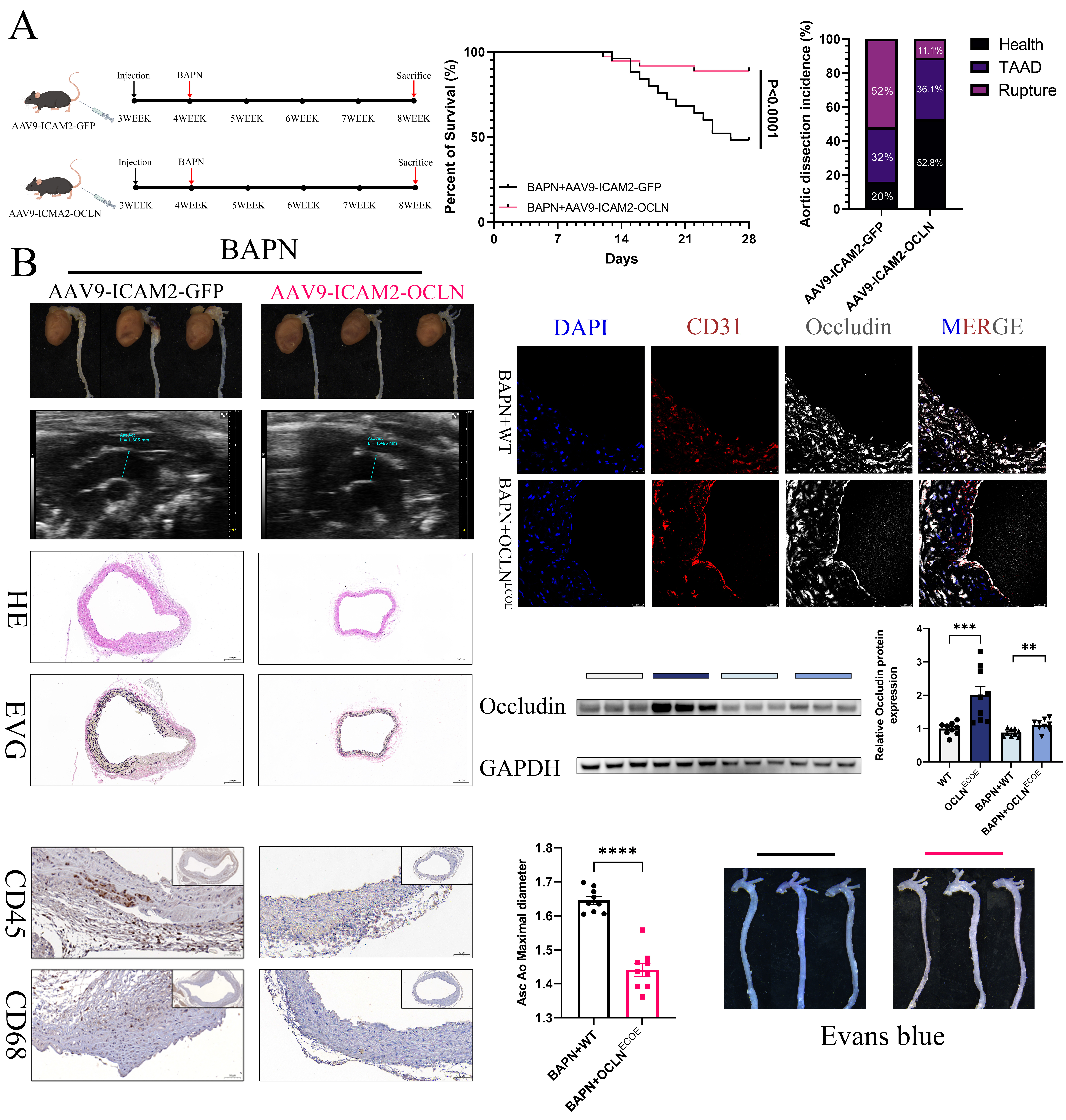
Single-cell RNA sequencing revealed aortic endothelial cell barrier dysfunction in human aortic dissection, in which the expression of the key target Occludin was reduced, and the reduced expression of aortic endothelial cell Occludin protein was verified by WB and immunofluorescence experiments in human and mouse aortic dissection tissue samples. Subsequently, Occludin protein was overexpressed by lentivirus and knocked down by small interfering RNA in human aortic endothelial cells (HAEC), and cell barrier function experiments showed that overexpression of Occludin protein decreased FITC-Dextran permeation, and knockdown of Occludin protein increased FITC-Dextran permeation. In the BAPN-induced AD mouse model, endothelial cell-specific knockdown of Occludin protein significantly increased mouse mortality, whereas endothelial cell-specific overexpression of Occludin protein significantly decreased mouse mortality. The upstream transcription factors of Occludin protein were predicted by KnockTF and JASPAR databases, and the interaction between upstream transcription factor KLF2 and Occludin was subsequently verified by yeast single heterozygote assay with dual luciferase gene reporter assay.
Conclusions
Endothelial cell dysfunction mediated by reduced endothelial cell Occludin protein expression is an important cause of aortic dissection.
Aortic dissection (AD) remains a significant challenge in cardiovascular medicine. Previous studies have found that reduced endothelial cell tight junction ZO-1 protein expression is associated with aortic dissection disease, but it is not clear whether the pattern of Occludin protein expression is the same.
Aims
The aim of this study was to investigate the potential role of aortic endothelial cell Occludin protein in aortic dissection.
Methods
Single-cell RNA sequencing was performed on the ascending aorta of five patients with AD and three heart transplant donors. Additionally, Crispr-cas9 technology and adeno-associated virus 9 (AAV9) were used to generate mouse models with endothelial cell-specific knockout and overexpression of the Ocln gene. Exploration and validation of transcription factors regulating the OCLN gene by dual luciferase gene reporter assay and yeast one-hybrid assay.
Results
Single-cell RNA sequencing revealed aortic endothelial cell barrier dysfunction in human aortic dissection, in which the expression of the key target Occludin was reduced, and the reduced expression of aortic endothelial cell Occludin protein was verified by WB and immunofluorescence experiments in human and mouse aortic dissection tissue samples. Subsequently, Occludin protein was overexpressed by lentivirus and knocked down by small interfering RNA in human aortic endothelial cells (HAEC), and cell barrier function experiments showed that overexpression of Occludin protein decreased FITC-Dextran permeation, and knockdown of Occludin protein increased FITC-Dextran permeation. In the BAPN-induced AD mouse model, endothelial cell-specific knockdown of Occludin protein significantly increased mouse mortality, whereas endothelial cell-specific overexpression of Occludin protein significantly decreased mouse mortality. The upstream transcription factors of Occludin protein were predicted by KnockTF and JASPAR databases, and the interaction between upstream transcription factor KLF2 and Occludin was subsequently verified by yeast single heterozygote assay with dual luciferase gene reporter assay.
Conclusions
Endothelial cell dysfunction mediated by reduced endothelial cell Occludin protein expression is an important cause of aortic dissection.
More abstracts on this topic:
Challenging Case: Acute Type A Aortic Dissection with Multi-Organ Malperfusion
Agarwal Rishab, Bradshaw Alleabelle, Lawton Jennifer
Aldosterone Promotes Aortic Dissection through Lactate/Lactylation-mediated Phenotypic Switching of Vascular Smooth Muscle CellLi Nanfang, Zhu Qing