Final ID: MP2633
Patient Preferences for Antithrombotic Therapy for Stroke Prevention in Device-Detected Subclinical Atrial Fibrillation: A Probability Trade-Off Interview Study
Abstract Body (Do not enter title and authors here): Introduction
Device-detected subclinical atrial fibrillation (AF) is common in patients with implanted cardiac rhythm devices. The ARTESiA trial demonstrated that in patients with subclinical AF, apixaban, as compared to aspirin, reduced stroke (0.98% versus 2.25% per year, 5 fewer strokes in 4 years with CHA2DS2-VASc > 4). However, apixaban also increased major bleeding (2.13% vs 1.45% per year, 2.72 more bleeds in 4 years with CHA2DS2-VASc > 4). Our objective was to estimate patient thresholds for stroke prevention and bleeding avoidance, as these factors may influence treatment decisions.
Methods
Trained interviewers enrolled patients with a CHA2DS2-VASc score ≥ 4 (irrespective of AF history) from a tertiary care pacemaker/defibrillator clinic. Participants underwent a structured interview with a probability trade-off tool. We determined risk thresholds for the minimum 4-year reduction in stroke necessary to prefer apixaban compared to aspirin (minimal important difference, stroke MID) and the maximum tolerable number of major bleeds to prevent one stroke (maximum allowable difference, bleed MAD).
We grouped participants into one of four preference clusters: stroke averse (accepting of bleeds to prevent stroke), bleeding averse (accepting of stroke to prevent bleeds), realist (accepting of stroke or bleed) and idealist (unwilling to accept stroke or bleed).
Results
Among 415 individuals approached, 300 participants consented and 275 completed the full interview. Mean age was 81.5 ± 6.9 years, 55.3% were female, median CHA2DS2-VASc score was 4 (IQR 4-5), and 148 (53.8%) had a history of AF.
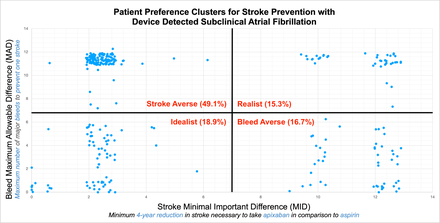
The overall mean stroke MID was 5.0 ± 4.5, meaning that on average, patients require a 5% reduction in stroke over 4 years of follow up to justify apixaban over aspirin. The overall bleed MAD was 8.0 ± 4.0, meaning that on average, patients were willing to endure 8 additional major bleeds to prevent one stroke. The highest proportion of participants were stroke averse (49.1%); a minority were bleeding averse (16.7%), realist (15.3%) and idealist (18.9%) [Figure].
Conclusions
Among patients with a cardiac rhythm device and CHA2DS2-VASc ≥ 4, the mean stroke risk reduction to justify the increased bleeding risk on apixaban, as compared to aspirin, is in line with the reduction in stroke seen for patients with subclinical AF in ARTESiA. Patients will accept a mean 8 additional bleeds to prevent one stroke. Patients are 3 times more likely to be stroke averse than bleeding averse.
Device-detected subclinical atrial fibrillation (AF) is common in patients with implanted cardiac rhythm devices. The ARTESiA trial demonstrated that in patients with subclinical AF, apixaban, as compared to aspirin, reduced stroke (0.98% versus 2.25% per year, 5 fewer strokes in 4 years with CHA2DS2-VASc > 4). However, apixaban also increased major bleeding (2.13% vs 1.45% per year, 2.72 more bleeds in 4 years with CHA2DS2-VASc > 4). Our objective was to estimate patient thresholds for stroke prevention and bleeding avoidance, as these factors may influence treatment decisions.
Methods
Trained interviewers enrolled patients with a CHA2DS2-VASc score ≥ 4 (irrespective of AF history) from a tertiary care pacemaker/defibrillator clinic. Participants underwent a structured interview with a probability trade-off tool. We determined risk thresholds for the minimum 4-year reduction in stroke necessary to prefer apixaban compared to aspirin (minimal important difference, stroke MID) and the maximum tolerable number of major bleeds to prevent one stroke (maximum allowable difference, bleed MAD).
We grouped participants into one of four preference clusters: stroke averse (accepting of bleeds to prevent stroke), bleeding averse (accepting of stroke to prevent bleeds), realist (accepting of stroke or bleed) and idealist (unwilling to accept stroke or bleed).
Results
Among 415 individuals approached, 300 participants consented and 275 completed the full interview. Mean age was 81.5 ± 6.9 years, 55.3% were female, median CHA2DS2-VASc score was 4 (IQR 4-5), and 148 (53.8%) had a history of AF.
The overall mean stroke MID was 5.0 ± 4.5, meaning that on average, patients require a 5% reduction in stroke over 4 years of follow up to justify apixaban over aspirin. The overall bleed MAD was 8.0 ± 4.0, meaning that on average, patients were willing to endure 8 additional major bleeds to prevent one stroke. The highest proportion of participants were stroke averse (49.1%); a minority were bleeding averse (16.7%), realist (15.3%) and idealist (18.9%) [Figure].
Conclusions
Among patients with a cardiac rhythm device and CHA2DS2-VASc ≥ 4, the mean stroke risk reduction to justify the increased bleeding risk on apixaban, as compared to aspirin, is in line with the reduction in stroke seen for patients with subclinical AF in ARTESiA. Patients will accept a mean 8 additional bleeds to prevent one stroke. Patients are 3 times more likely to be stroke averse than bleeding averse.
More abstracts on this topic:
10-Year Trend Analysis of Medicare Payment in Stroke Inpatient Hospital Admission
Wong Ka-ho, Krothapalli Neeharika, Littig Lauren, Champagne Alison, Majersik Jennifer, Reddy Vivek, De Havenon Adam
An Evaluation of Stroke Literature Pamphlets For Stroke PatientsDegen Nathaniel, Sivakumar Milan, Varkey Thomas, Alexandrov Andrei, Singh Savdeep