Final ID: MP2496
Use Of Glucagon Like Peptide 1 Receptor Agonists In Non Diabetic Patients With Dilated Cardiomyopathy
Abstract Body (Do not enter title and authors here): Background:
Glucagon-like peptide 1 (GLP-1) receptor agonists have demonstrated cardiovascular benefits in diabetic patients. However, their role in patients with dilated cardiomyopathy (DCM) without diabetes remains unclear.
Methods:
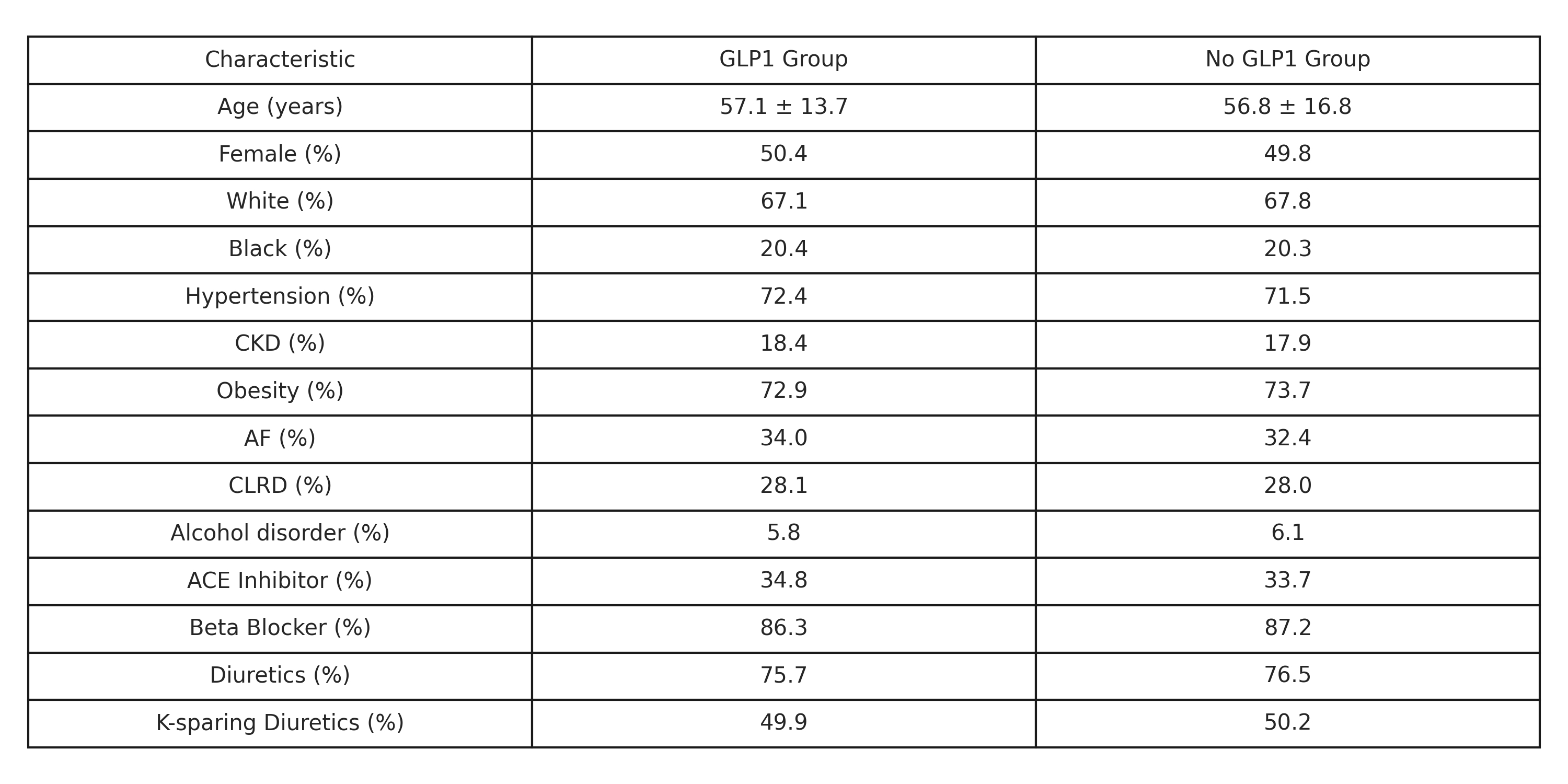
We conducted a real-world, retrospective cohort study using the TriNetX Global Collaborative Network. Adults aged 18 or older with a diagnosis of DCM (ICD-10 I42.0) and no diagnosis of diabetes (ICD-10 E10–E13) were identified. Patients were stratified based on GLP-1 receptor agonist use (liraglutide, semaglutide, dulaglutide, lixisenatide, tirzepatide, pramlintide). Propensity score matching (1:1) was performed to balance demographics, comorbidities, and cardiovascular medications, yielding 2,871 patients per group. Outcomes were assessed from 1 day to 365 days after the index event. The primary outcome was all-cause mortality; secondary outcomes included hospitalization, myocardial infarction (MI), and heart failure exacerbation (HF-exa). Median follow-up time was approximately 11 months in both groups.
Results:
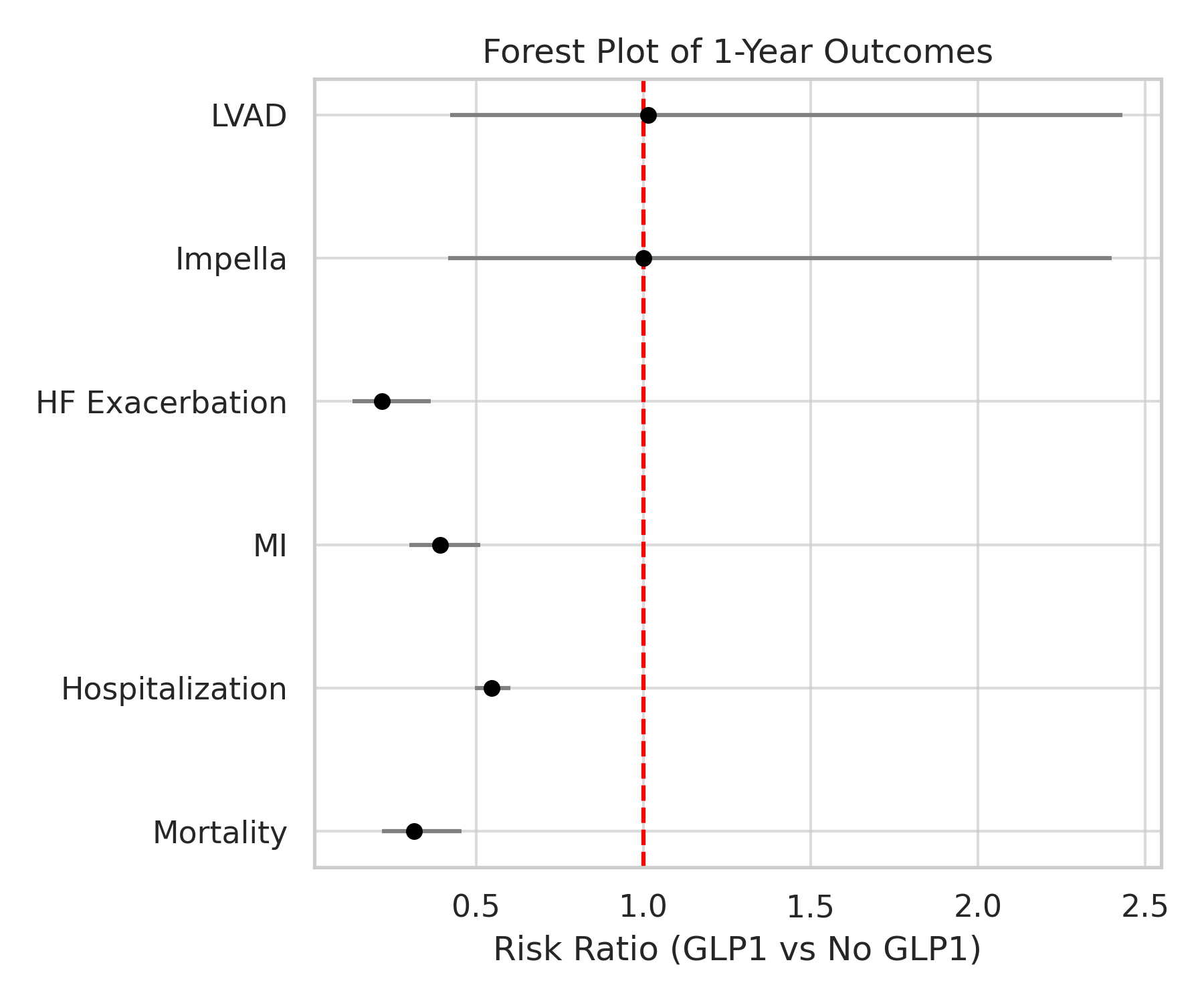
GLP-1 receptor agonist use was associated with significantly lower risks of all-cause mortality (1.3% vs 4.1%; risk ratio [RR] 0.316; 95% CI 0.219–0.456; p<0.001), hospitalization (17.5% vs 32.0%; RR 0.547; p<0.001), MI (2.5% vs 6.5%; RR 0.392; p<0.001), and HF-exa (0.8% vs 3.7%; RR 0.220; p<0.001). Kaplan-Meier analysis confirmed higher 1-year survival probabilities and lower event rates in the GLP-1 group across all endpoints. No significant differences were observed in use of mechanical circulatory support devices (LVAD or Impella).
Conclusion:
In this real-world, propensity-matched study of non-diabetic patients with DCM, GLP-1 receptor agonists were associated with substantial reductions in 1-year mortality, hospitalization, MI, and HF exacerbation. Although a 3-year follow-up window was available, median follow-up (~11 months) supported 1 year as the most reliable endpoint. Limitations include lack of LVEF or biomarker data, unmeasured confounding, and inability to confirm long-term medication adherence or persistence. Additionally, GLP-1 use may reflect closer outpatient monitoring or healthier behaviors not captured in the dataset. These findings warrant prospective trials to evaluate GLP-1 receptor agonists as cardioprotective therapies in heart failure beyond glycemic control.
Glucagon-like peptide 1 (GLP-1) receptor agonists have demonstrated cardiovascular benefits in diabetic patients. However, their role in patients with dilated cardiomyopathy (DCM) without diabetes remains unclear.
Methods:
We conducted a real-world, retrospective cohort study using the TriNetX Global Collaborative Network. Adults aged 18 or older with a diagnosis of DCM (ICD-10 I42.0) and no diagnosis of diabetes (ICD-10 E10–E13) were identified. Patients were stratified based on GLP-1 receptor agonist use (liraglutide, semaglutide, dulaglutide, lixisenatide, tirzepatide, pramlintide). Propensity score matching (1:1) was performed to balance demographics, comorbidities, and cardiovascular medications, yielding 2,871 patients per group. Outcomes were assessed from 1 day to 365 days after the index event. The primary outcome was all-cause mortality; secondary outcomes included hospitalization, myocardial infarction (MI), and heart failure exacerbation (HF-exa). Median follow-up time was approximately 11 months in both groups.
Results:
GLP-1 receptor agonist use was associated with significantly lower risks of all-cause mortality (1.3% vs 4.1%; risk ratio [RR] 0.316; 95% CI 0.219–0.456; p<0.001), hospitalization (17.5% vs 32.0%; RR 0.547; p<0.001), MI (2.5% vs 6.5%; RR 0.392; p<0.001), and HF-exa (0.8% vs 3.7%; RR 0.220; p<0.001). Kaplan-Meier analysis confirmed higher 1-year survival probabilities and lower event rates in the GLP-1 group across all endpoints. No significant differences were observed in use of mechanical circulatory support devices (LVAD or Impella).
Conclusion:
In this real-world, propensity-matched study of non-diabetic patients with DCM, GLP-1 receptor agonists were associated with substantial reductions in 1-year mortality, hospitalization, MI, and HF exacerbation. Although a 3-year follow-up window was available, median follow-up (~11 months) supported 1 year as the most reliable endpoint. Limitations include lack of LVEF or biomarker data, unmeasured confounding, and inability to confirm long-term medication adherence or persistence. Additionally, GLP-1 use may reflect closer outpatient monitoring or healthier behaviors not captured in the dataset. These findings warrant prospective trials to evaluate GLP-1 receptor agonists as cardioprotective therapies in heart failure beyond glycemic control.
More abstracts on this topic:
A Novel Classification of Heart Failure Derived from the Nationwide JROADHF Cohort Using Unsupervised Machine Learning
Kyodo Atsushi, Tsutsui Hiroyuki, Hikoso Shungo, Nakada Yasuki, Nogi Kazutaka, Ishihara Satomi, Ueda Tomoya, Tohyama Takeshi, Enzan Nobuyuki, Matsushima Shouji, Ide Tomomi
6-Nitrodopamine potentiates the positive chronotopic and inotropic effect induced by noradrenaline in the rat isolated heartLima Antonio, Sobanski Joao Fernando, Antunes Edson, De Nucci Gilberto