Final ID: MP1433
Intensive Versus Standard Blood Pressure Control in Patients with Type 2 Diabetes Mellitus: An Updated Systematic Review and Meta-analysis
Abstract Body (Do not enter title and authors here): Background: Hypertension (HTN), a prevalent comorbidity in type 2 diabetes mellitus (T2DM), increases the risk of cardiovascular (CV) events, mortality, and kidney complications. However, optimal blood pressure (BP) targets in patients with T2DM remain unclear.
Research Question: Does intensive BP control, compared to standard targets, reduce CV, renal, and mortality outcomes in patients with T2DM?
Aims: We aim to conduct an updated meta-analysis to evaluate the effects of intensive vs. standard BP control on CV and kidney outcomes and mortality in T2DM patients.
Methods: A comprehensive search of PubMed, Cochrane Library, and Scopus was conducted through December 2024 for trials comparing intensive vs. standard BP control in patients with T2DM. Outcomes assessed included all-cause mortality, CV mortality, major adverse CV events (MACE), stroke, myocardial infarction (MI), incident heart failure (HF), chronic kidney disease (CKD) development, albuminuria, and serious adverse events (SAEs). Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using a random effects model.
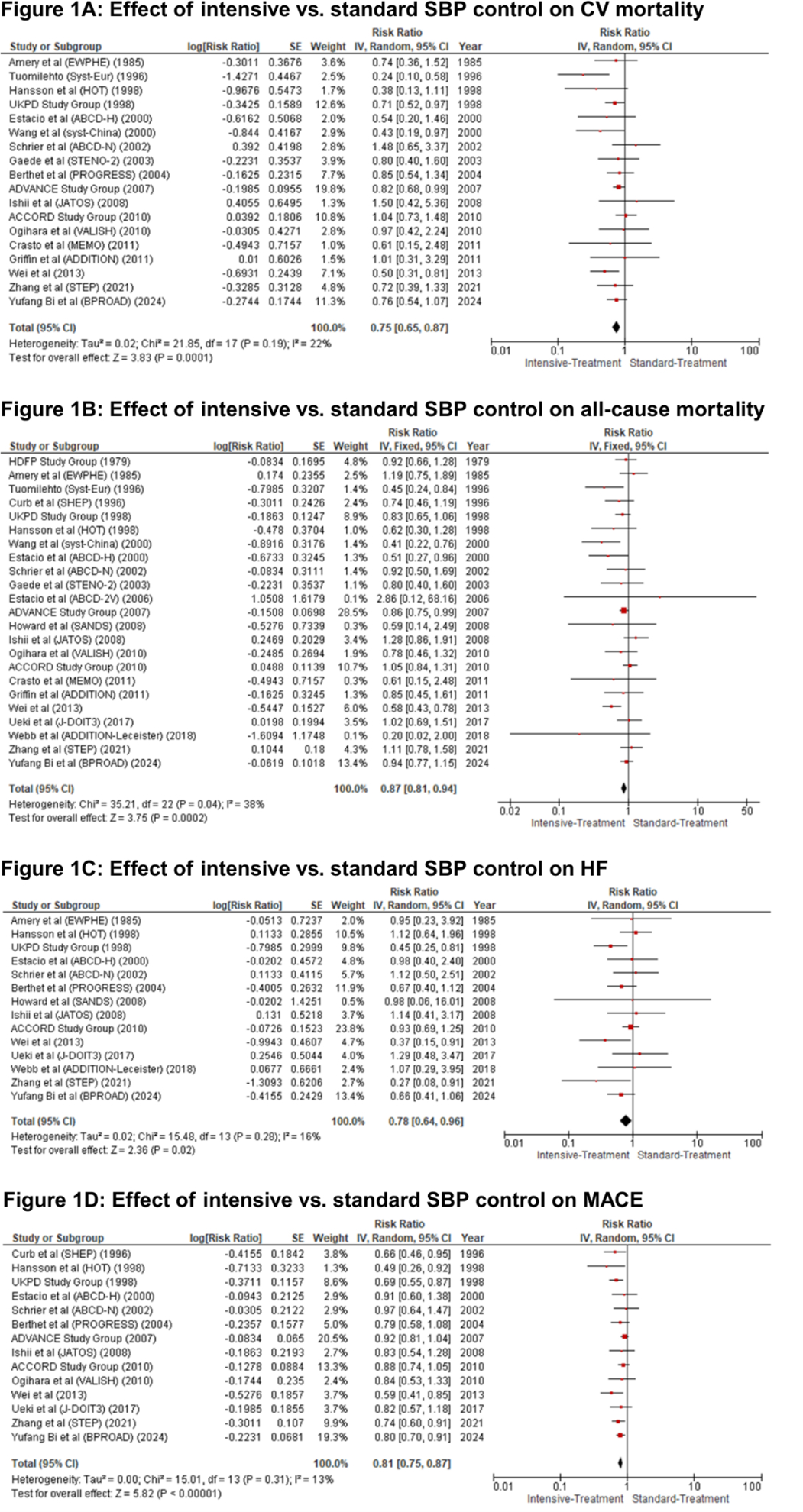
Results: 28 trials encompassing 104,634 patients were included. Intensive BP control significantly reduced the risk of CV mortality (RR: 0.75, 95% CI: 0.65–0.87, P = 0.0001), all-cause mortality (RR: 0.85, 95% CI: 0.76–0.95, P = 0.004), MACE (RR: 0.81, 95% CI: 0.75–0.87, P < 0.00001), stroke (RR: 0.70, 95% CI: 0.61–0.80, P < 0.00001), MI (RR: 0.86, 95% CI: 0.79–0.94, P = 0.001), HF (RR: 0.78, 95% CI: 0.64–0.96, P = 0.02), and albuminuria (RR: 0.89, 95% CI: 0.82–0.97, P = 0.005). There were no significant differences in CKD development (RR: 1.08, 95% CI: 0.92–1.26, P = 0.36) or SAEs (RR: 1.16, 95% CI: 0.97–1.40, P = 0.10).
Conclusions: Intensive BP control in patients with T2DM was associated with a lower risk of all-cause mortality, CV mortality, MACE, stroke, MI, HF, and albuminuria as compared to standard control, without an increased risk of serious adverse events.
Research Question: Does intensive BP control, compared to standard targets, reduce CV, renal, and mortality outcomes in patients with T2DM?
Aims: We aim to conduct an updated meta-analysis to evaluate the effects of intensive vs. standard BP control on CV and kidney outcomes and mortality in T2DM patients.
Methods: A comprehensive search of PubMed, Cochrane Library, and Scopus was conducted through December 2024 for trials comparing intensive vs. standard BP control in patients with T2DM. Outcomes assessed included all-cause mortality, CV mortality, major adverse CV events (MACE), stroke, myocardial infarction (MI), incident heart failure (HF), chronic kidney disease (CKD) development, albuminuria, and serious adverse events (SAEs). Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using a random effects model.
Results: 28 trials encompassing 104,634 patients were included. Intensive BP control significantly reduced the risk of CV mortality (RR: 0.75, 95% CI: 0.65–0.87, P = 0.0001), all-cause mortality (RR: 0.85, 95% CI: 0.76–0.95, P = 0.004), MACE (RR: 0.81, 95% CI: 0.75–0.87, P < 0.00001), stroke (RR: 0.70, 95% CI: 0.61–0.80, P < 0.00001), MI (RR: 0.86, 95% CI: 0.79–0.94, P = 0.001), HF (RR: 0.78, 95% CI: 0.64–0.96, P = 0.02), and albuminuria (RR: 0.89, 95% CI: 0.82–0.97, P = 0.005). There were no significant differences in CKD development (RR: 1.08, 95% CI: 0.92–1.26, P = 0.36) or SAEs (RR: 1.16, 95% CI: 0.97–1.40, P = 0.10).
Conclusions: Intensive BP control in patients with T2DM was associated with a lower risk of all-cause mortality, CV mortality, MACE, stroke, MI, HF, and albuminuria as compared to standard control, without an increased risk of serious adverse events.
More abstracts on this topic:
A Meta-Analysis Comparing Same-Day Discharge to Later-Day Discharge in Transcatheter Aortic Valve Replacement
Jain Hritvik, Passey Siddhant, Jain Jyoti, Goyal Aman, Wasir Amanpreet, Ahmed Mushood, Patel Nandan, Yadav Ashish, Shah Janhvi, Mehta Aryan
A Diagnosis Dilemma of Positional Hypoxia: Scoliosis-Mediated Platypnea-Orthodeoxia SyndromeAdemuwagun Christianah, Arjoon Roy, Seth Paula, Chang Gene, Ibe Oby