Final ID: 4360699
Hemodynamic Regulation of FOXO1 Integrates Endothelial Inflammation and Metabolism in Atherosclerosis
Abstract Body (Do not enter title and authors here): Background:
Atherosclerosis occurs preferentially in regions of disturbed or low fluid shear stress (FSS), whereas physiological laminar FSS protects against disease by suppressing endothelial inflammation. Pro- vs anti-inflammatory programs are associated with glycolysis vs mitochondrial metabolism, respectively. Endothelial cells (ECs) sensing FSS from blood flow regulates these responses, but the underlying mechanisms are poorly understood. The transcription factor Forkhead box protein O1 (FOXO1) is known to regulate endothelial metabolism, yet its role in FSS-regulated endothelial inflammation remains largely unclear.
Methods:
In vitro, ECs were subjected to defined flow patterns using a parallel plate flow chamber. Immunofluorescence, RNA sequencing, and biochemical assays were used to evaluate FOXO1 localization, gene expression, and post-translational modifications. In vivo experiments used FOXO1 floxed mice crossed with Bmx-CreERT2 for artery ECs-specific FOXO1 knockout. Hyperlipidemia was induced via injection of PCSK9 adeno-associated virus and high-cholesterol/high-fat diet (HCHFD) to assess atherosclerosis.
Results:
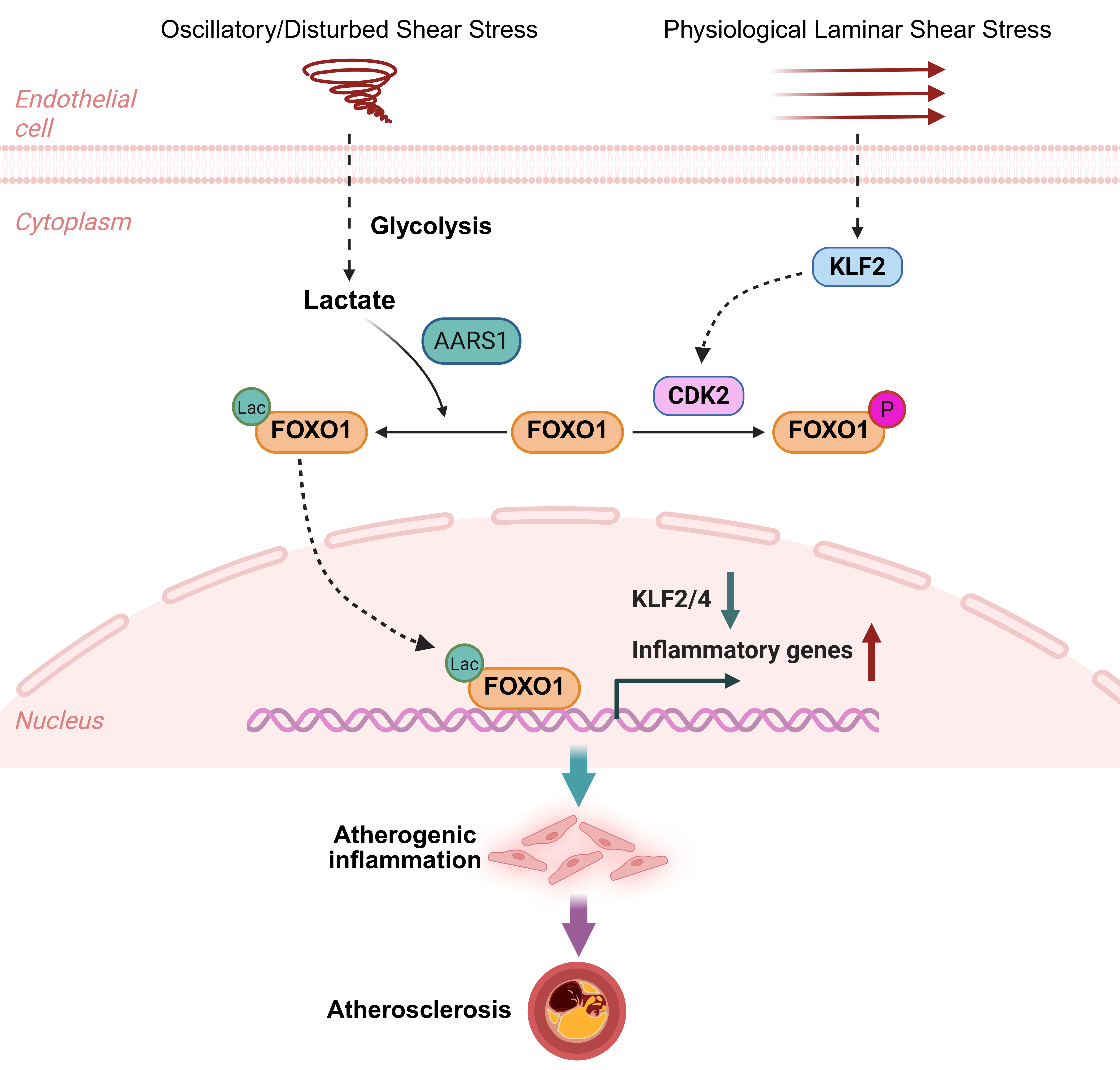
Oscillatory FSS and inflammatory cytokines induce, whereas physiological FSS inhibits FOXO1 nuclear translocation. RNA sequencing revealed that FOXO1 depletion in ECs upregulates the protective flow-responsive transcription factors KLF2/4 and reduces oscillatory FSS-induced inflammatory genes. Inhibition of FOXO1 nuclear translocation by physiological FSS is mediated via a KLF2-CDK2 pathway, with the latter phosphorylating FOXO1 at S249. Artery ECs-specific deletion of FOXO1 significantly reduces atherosclerotic plaques formation in hyperlipidemic mice. Inhibition of glycolysis attenuates OSS-induced FOXO1 nucleus translocation, suggesting metabolic regulation. Notably, treatment with lactate promotes FOXO1 nuclear localization and lactylation, which is mediated by a lactyltransferase AARS1 (Alanyl-tRNA synthetase).
Conclusions:
These findings identify FOXO1 as a key mediator linking atheroprone flow and endothelial inflammation via lactate-driven nuclear translocation and lactylation, promoting atherosclerosis. Conversely, physiological FSS suppresses FOXO1 via KLF2-CDK2 signaling. These complementary pathways suggest potential new therapeutic targets for treating atherosclerotic cardiovascular disease.
Atherosclerosis occurs preferentially in regions of disturbed or low fluid shear stress (FSS), whereas physiological laminar FSS protects against disease by suppressing endothelial inflammation. Pro- vs anti-inflammatory programs are associated with glycolysis vs mitochondrial metabolism, respectively. Endothelial cells (ECs) sensing FSS from blood flow regulates these responses, but the underlying mechanisms are poorly understood. The transcription factor Forkhead box protein O1 (FOXO1) is known to regulate endothelial metabolism, yet its role in FSS-regulated endothelial inflammation remains largely unclear.
Methods:
In vitro, ECs were subjected to defined flow patterns using a parallel plate flow chamber. Immunofluorescence, RNA sequencing, and biochemical assays were used to evaluate FOXO1 localization, gene expression, and post-translational modifications. In vivo experiments used FOXO1 floxed mice crossed with Bmx-CreERT2 for artery ECs-specific FOXO1 knockout. Hyperlipidemia was induced via injection of PCSK9 adeno-associated virus and high-cholesterol/high-fat diet (HCHFD) to assess atherosclerosis.
Results:
Oscillatory FSS and inflammatory cytokines induce, whereas physiological FSS inhibits FOXO1 nuclear translocation. RNA sequencing revealed that FOXO1 depletion in ECs upregulates the protective flow-responsive transcription factors KLF2/4 and reduces oscillatory FSS-induced inflammatory genes. Inhibition of FOXO1 nuclear translocation by physiological FSS is mediated via a KLF2-CDK2 pathway, with the latter phosphorylating FOXO1 at S249. Artery ECs-specific deletion of FOXO1 significantly reduces atherosclerotic plaques formation in hyperlipidemic mice. Inhibition of glycolysis attenuates OSS-induced FOXO1 nucleus translocation, suggesting metabolic regulation. Notably, treatment with lactate promotes FOXO1 nuclear localization and lactylation, which is mediated by a lactyltransferase AARS1 (Alanyl-tRNA synthetase).
Conclusions:
These findings identify FOXO1 as a key mediator linking atheroprone flow and endothelial inflammation via lactate-driven nuclear translocation and lactylation, promoting atherosclerosis. Conversely, physiological FSS suppresses FOXO1 via KLF2-CDK2 signaling. These complementary pathways suggest potential new therapeutic targets for treating atherosclerotic cardiovascular disease.
More abstracts on this topic:
Alpha-ketoglutarate facilitates high-fat diet for inflammatory myeloid cell production and atherosclerotic plaque progression via OXGR1
Zhao Jiwei, Feng Yingmei
Abnormal day-night blood pressure and systemic hemodynamics in heart failure compared to normotension and controlled hypertensionSrungavarapu Sambasiva Rao, Khatri Chander, Osmond Peter, Izzo Joseph