Final ID: MP1454
Real World Evaluation of the Eligibility, Uptake, and Cardiometabolic Effects of Tirzepatide: An Analysis from the Platform for Evidence GeneraTion in CArdiometabolic HeaLth (PETAL) Study
Abstract Body (Do not enter title and authors here): Background: Tirzepatide is a dual glucose-dependent insulinotropic polypeptide and glucagon like peptide-1 receptor agonist that has been shown to have cardiometabolic benefits. However, the real-world eligibility, uptake, and population level impact remains unclear.
Research Question/Aims: To describe patients in the U.S. who meet an indication for tirzepatide, to understand its real-world uptake, and to estimate the population level impact on cardiometabolic risk factors if uptake were to increase.
Methods: A retrospective study using electronic medical record data from 5 US health systems in PCORnet® identified a cohort of adults between 3/31/2023-3/31/2024 who were obese (BMI of ≥30 kg/m2) or overweight (27 kg/m2 - < 30 kg/m2) with at least one weight-related co-morbidity). Effect estimates of cardiometabolic risk factor changes from the SURMOUNT-1 trial and SURPASS-2 trial were applied to estimate population-level impacts of tirzepatide on weight and other cardiometabolic risk factors.
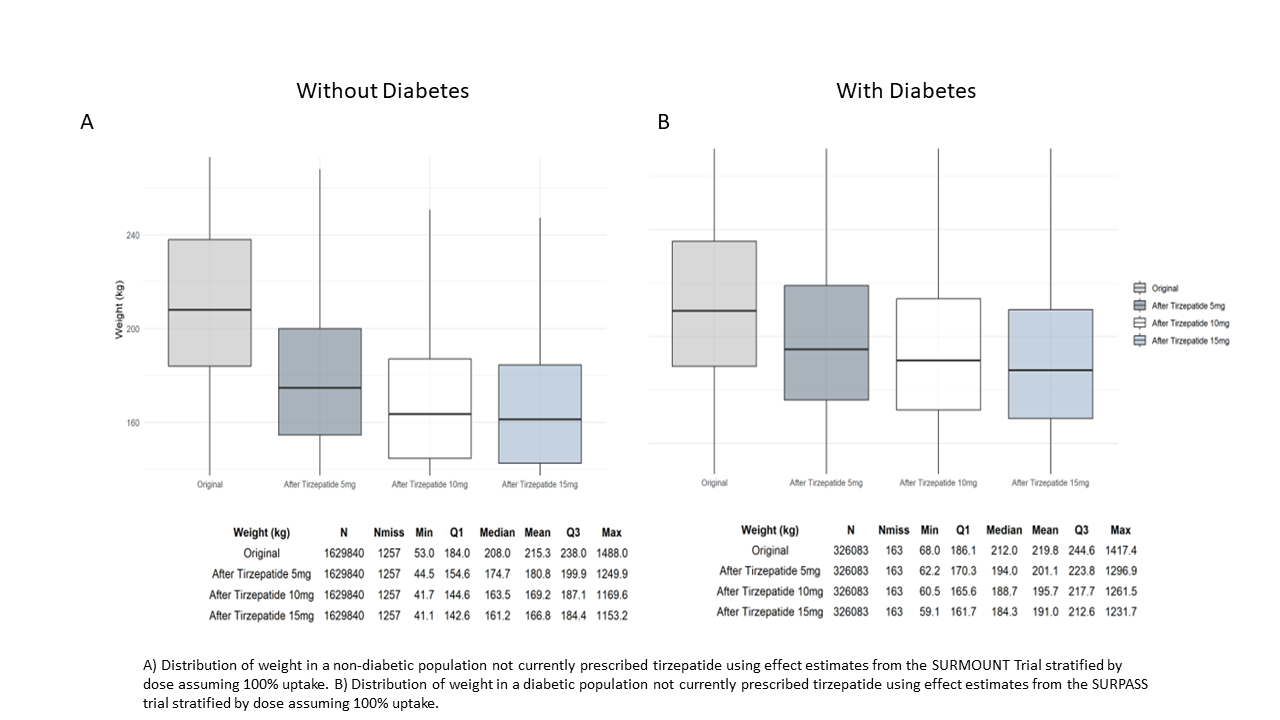
Results: A total of 2,092,393 individuals had obesity or were overweight with at least one weight related comorbidity. About 25% were overweight; and 39%, 20%, and 16% had class 1, 2 and 3 obesity, respectively. The mean age (SD) was 54 (17.1) years, 57.2% were female, 19.7% were Black or African American, 9.1% were of Hispanic ethnicity, and 83.6% had commercial or government insurance. Approximately 20% had diabetes, and 21% had 4 or more obesity-related comorbidities. Overall, 1.3% (n=26,577) were prescribed any dose of tirzepatide, including 3.9% of those with diabetes and 0.6% of those without diabetes. Among those with diabetes, 7.0% of those with commercial and 2.4% of those with government insurance were prescribed tirzepatide; among those without diabetes, the respective proportions were 0.9% and 0.4%. A substantial reduction in weight was estimated with tirzepatide. The figure shows estimated changes in population-level distribution of weight if tirzepatide (at varying doses) was used by all eligible patients, stratified by diabetes status. Changes in other cardiometabolic risk factors were also estimated to be improved.
Conclusions: Across 5 large US health systems, only 1.3% of eligible patients were prescribed tirzepatide. Greater uptake is projected to be associated with population-level improvements in weight and other cardiometabolic risk factors. There is a critical need to overcome access and other barriers to the use of effective obesity medications.
Research Question/Aims: To describe patients in the U.S. who meet an indication for tirzepatide, to understand its real-world uptake, and to estimate the population level impact on cardiometabolic risk factors if uptake were to increase.
Methods: A retrospective study using electronic medical record data from 5 US health systems in PCORnet® identified a cohort of adults between 3/31/2023-3/31/2024 who were obese (BMI of ≥30 kg/m2) or overweight (27 kg/m2 - < 30 kg/m2) with at least one weight-related co-morbidity). Effect estimates of cardiometabolic risk factor changes from the SURMOUNT-1 trial and SURPASS-2 trial were applied to estimate population-level impacts of tirzepatide on weight and other cardiometabolic risk factors.
Results: A total of 2,092,393 individuals had obesity or were overweight with at least one weight related comorbidity. About 25% were overweight; and 39%, 20%, and 16% had class 1, 2 and 3 obesity, respectively. The mean age (SD) was 54 (17.1) years, 57.2% were female, 19.7% were Black or African American, 9.1% were of Hispanic ethnicity, and 83.6% had commercial or government insurance. Approximately 20% had diabetes, and 21% had 4 or more obesity-related comorbidities. Overall, 1.3% (n=26,577) were prescribed any dose of tirzepatide, including 3.9% of those with diabetes and 0.6% of those without diabetes. Among those with diabetes, 7.0% of those with commercial and 2.4% of those with government insurance were prescribed tirzepatide; among those without diabetes, the respective proportions were 0.9% and 0.4%. A substantial reduction in weight was estimated with tirzepatide. The figure shows estimated changes in population-level distribution of weight if tirzepatide (at varying doses) was used by all eligible patients, stratified by diabetes status. Changes in other cardiometabolic risk factors were also estimated to be improved.
Conclusions: Across 5 large US health systems, only 1.3% of eligible patients were prescribed tirzepatide. Greater uptake is projected to be associated with population-level improvements in weight and other cardiometabolic risk factors. There is a critical need to overcome access and other barriers to the use of effective obesity medications.
More abstracts on this topic:
Cardiovascular and Rhythm Benefits of GLP-1 Receptor Agonists in Obese Patients: A Real-World Multicenter Study
Yousafzai Osman, Annie Frank, Kanwal Kainat, Mahmoud Mohamed, Rinehart Sarah
Circulating Metabolomic Biomarkers of 5-Year Unintentional Weight Loss in a Biracial Community-Dwelling Older CohortYao Shanshan, Marron Megan, Miljkovic Iva, Farsijani Samaneh, Tseng George, Shah Ravi, Murthy Venkatesh, Newman Anne